Effect of QPQ Nitriding Process On Thickness of 42CrMo Piston Rod
The effect of salt bath nitriding on the properties of the permeable layer is mainly manifested in the hardness, wear resistance and corrosion resistance. In the hardness test, the orthogonal test method is developed according to reasonable process parameters, as shown in Table 2.2. The hardness test obtains the core hardness of specimens, and then obtains the penetration layer thickness from the core hardness. Finally, the index of penetration layer thickness and surface hardness is obtained. The research on wear resistance mainly uses the change of wear amount and friction coefficient as experimental indexes to obtain the influence degree of different process parameters on wear resistance of penetration layer and the optimal scheme. Electrochemical corrosion experiments are mainly used to study the corrosion resistance, in which two slopes are adopted: anode tower fir slope Ba/mV and cathode tower Fir slope Bc/mV. Two special parameters, corrosion potential Eo/volts and corrosion current density Io/(cm2), are used as electrochemical parameters. Finally, the influence degree of different process parameters on the corrosion resistance of the infiltration layer and the optimal scheme are obtained. Figure 5.1 shows the cyclic polarization curve [52].
(1) When the regression scanning curve is shown in Figure Path1, it indicates that the material is immune to corrosion and corrosion will not occur at all.
(2) When the regression scanning curve is shown in Figure Path2, the corrosion current density increases suddenly, which will accelerate the corrosion. However, due to the existence of passivation film on the surface of the specimen, the specimen has a certain self-healing ability.
(3) If Path has poor or no self-repair ability, serious corrosion will occur. The cyclic polarization curves of QPQ salt bath nitriding specimens were analyzed, and two slopes and two special performance parameters in electrochemical corrosion were obtained by electrochemical analysis software.
1. Effect of QPQ salt bath nitriding on hardness of 42CrMo steel infiltration layer
In the experiment, HX-1000TM microhardness tester was used to test the hardness of 42CrMo steel samples. The penetration layer thickness is the core hardness plus the hardness value of 30HV. The depth of the corresponding position is the penetration layer thickness. Through the cyanate concentration, nitriding temperature, nitriding time and other three orthogonal factors 9 times orthogonal experiment, obtained QPQ salt bath nitriding 42CrMo steel hardness value, so as to obtain the infiltration layer thickness of each group of experiments.
1.1 Hardness of 42CrMo steel penetration layer
Figure 5.2 and 5.3 show the hardness test data of 9 groups of QPQ salt bath nitriding specimens. It can be seen from Figure 5.2 and 5.3 that: (a) the permeability layer thickness of group parameters is about 141um; (b) The permeability layer thickness of group parameters is about 252um; (c) The penetration thickness of group parameters is about 224um; (d) The penetration thickness of group parameters is about 227um; (e) The permeability layer thickness of group parameters is about 247um; (f) The permeability layer thickness of group parameters is about 235um; (g) The penetration thickness of group parameters is about 226um; (h) group parameters of infiltration layer thickness is about 243um; The permeability thickness of group (i) parameters is about 241um. In general, the hardness reaches the maximum value when the distance from the surface is 10um, and the hardness value decreases when measured from the surface of the specimen to the core of the specimen, and finally reaches the corresponding core hardness value. Therefore, the hardness index values analyzed by the 9 groups of orthogonal experiments all used the hardness values with a distance of 10um from the surface.
1.2 Orthogonal analysis of 42CrMo steel penetration layer thickness
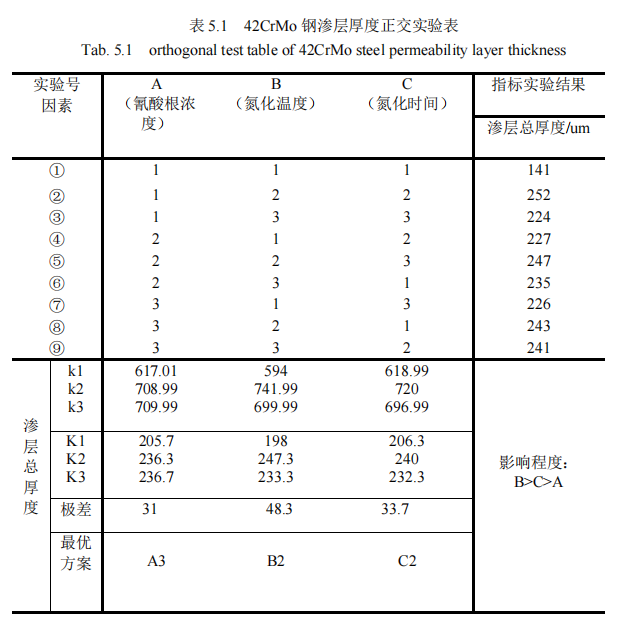
According to the hardness gradient variation figure 5.2 and Figure 5.3 of QPQ salt bath nitriding 42CrMo steel, the permeability layer thickness of test specimens in each group can be obtained. The orthogonal analysis of permeability layer thickness is shown in Table 5.2.
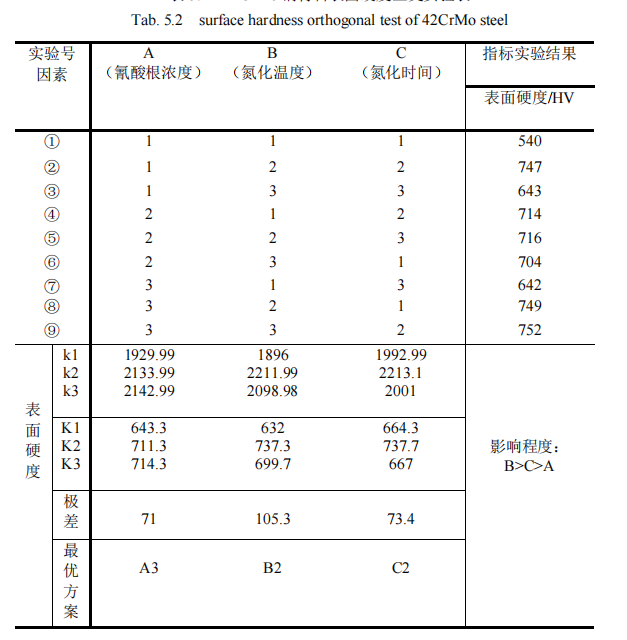
It can be seen from the standard orthogonal test in Table 5.2 that when three different factors and three different levels are set, the permeability layer thickness changes significantly. The range of the three factors is given in the table as 31, 48.3, and 33.7 respectively. Obviously, it can be concluded that the range of the nitriding temperature in the second column is the largest, which indicates that among the process parameters, The change of nitriding temperature has the greatest influence on the thickness of the experimental infiltration layer, so the nitriding temperature is the main factor we should consider, and the experimental index is the thickness of the infiltration layer, the bigger the better, and the largest of its three levels K1, K2 and K3 is K2=247.3, so the second level is the best. Secondly, the range value of the nitriding time in the third column is 33.7, which indicates that among the process parameters, the change of the factor of nitriding time has a great influence on the thickness of the experimental infiltration layer. Therefore, the factor of nitriding time is the second important factor to be considered, and the largest of its three levels K1, K2 and K3 is K2=240. So it’s best to take the second level. Finally, it is analyzed that the range value 31 of cyanate concentration in the first column is the smallest, which indicates that the change of cyanate concentration level has the smallest influence on experimental indexes. Moreover, among the three levels K1, K2 and K3, the largest is K3=236.7, so the third level is the best. Based on the above results, the influence degree of the three factors is B> C> A, the nitriding temperature > Nitriding time > Cyanate concentration. The optimal solution was A3B2C2, i.e., 32% cyanate concentration, 570℃ nitriding temperature and 120min nitriding time.
1.3 Orthogonal analysis of 42CrMo steel surface hardness
After the QPQ salt bath nitride treatment, different parameters will directly affect the tissue formation of the permeability layer of the specimen, thus affecting the hardness value of the workpiece surface. The hardness value at the distance of 10um from the surface is taken as the surface hardness index of the specimen, and a set of standard orthogonal test scheme is designed, as shown in Table 5.2.
In the orthogonal test table shown in Table 5.2, when three different factors and three different levels are set, the surface hardness value of the infiltration layer at 10um changes significantly. The table shows that the range of the three factors is 71, 105.3 and 73.4, respectively. Obviously, it can be concluded that the range of the nitriding temperature in the second column is 105.3, which is the largest. This indicates that the change of nitriding temperature has the greatest influence on the surface hardness index, so the nitriding temperature is the main factor to be considered in this paper, while the experimental index is the material surface hardness, the greater the value, the better, and the largest of its three levels K1, K2 and K3 is K2=737.3, so the second level is the best. Secondly, the range value of the nitriding time in the third column is 73.4, which indicates that the change of the nitriding time level has a great influence on the hardness index of the material surface. Therefore, the factor of nitriding time is the second important factor to be considered in this paper, and the largest of its three levels K1, K2 and K3 is K2=737.7, so the second level is the best. Finally, it is analyzed that the range value 71 of cyanate concentration in the first column is the smallest, which indicates that the change of cyanate concentration level has the smallest influence on the experimental index. Moreover, among its three levels K1, K2 and K3, the largest is K3=714.3, so the third level is the best. Based on the above results, the influence degree of the three factors is B> C> A, the nitriding temperature > Nitriding time > Cyanate concentration. The optimal solution was A3B2C2, i.e., 32% cyanate concentration, 570℃ nitriding temperature and 120min nitriding time.
2. 42CrMo steel surface electrochemical corrosion experiment
Experimental results of 42CrMo steel surface electrochemistry
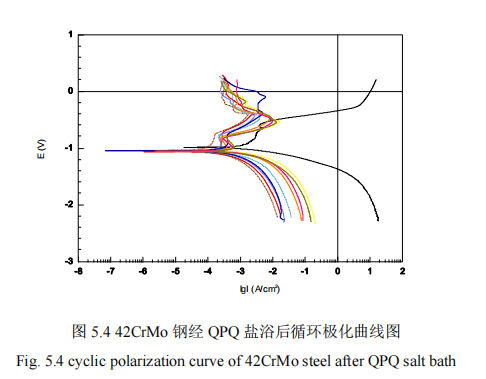
In Figure 5.4, the black solid line at the far right is the cyclic polarization curve of 42CrMo steel matrix material, and the dotted line at the left is the cyclic polarization curve of nine orthogonal groups.
It can be seen from the data in Figure 5.4 that after the 42CrMo steel is nitrized by QPQ salt bath, different process parameters show different corrosion resistance, but the corrosion resistance of 9 orthogonal experimental groups is similar. In the figure as a whole, after QPQ salt bath nitriding parts than 42CrMo steel matrix material self-corrosion potential and rupture potential has been greatly improved, and the corrosion current density is reduced, which indicates that after QPQ salt bath nitriding, improve the corrosion resistance of the specimen, reduce the corrosion rate.
Below, nine groups of electrochemical corrosion Tabfil fitting results will be obtained from the electrochemical analysis software to obtain the corrosion resistance energy parameter values, and then the data will be summarized, as shown in Table 5.3.
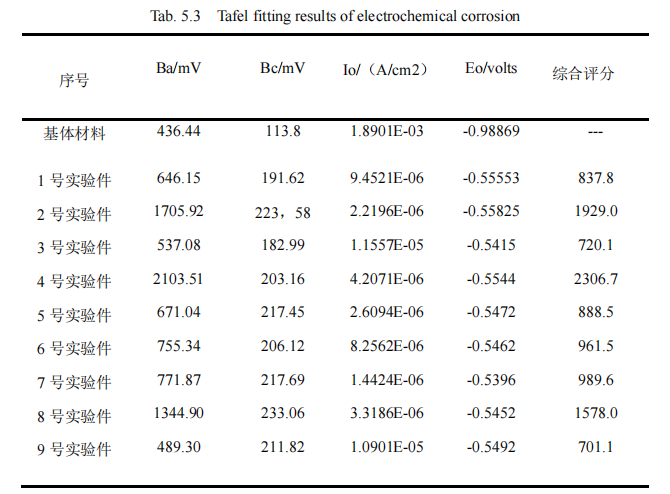
Tab.5.3 shows the Tab.3 fitting results of electrochemical corrosion experiments. The indexes include two kinds of slopes: anode Tab.5 slope Ba/mV and cathode tab.6 slope Bc/mV. Two important performance parameters: corrosion potential Eo/volts and corrosion current density Io/(cm2). It can be seen from the table that after QPQ salt bath nitridation, the positive/cathode tafel slope and corrosion potential values are significantly increased, and the corrosion current value is significantly decreased, which indicates that the corrosion resistance of the specimen has been improved. Combined with reference 4, according to the comparison of the corrosion resistance data of QPQ and chrome plating, these four electrochemical performance parameters are of the same importance, and the larger the anode tower slope, cathode tower slope and corrosion potential value, the smaller the corrosion current density value, indicating that the corrosion resistance of the specimen is better. Therefore, the comprehensive score = anode Taffel slope + cathode Taffel slope + corrosion potential – corrosion current density. Due to the large difference in experimental data, the comprehensive score is the approximate value of the total score, which is used to conduct the orthogonal experiment of corrosion.
2.1 Orthogonal analysis of 42CrMo steel electrochemical corrosion experiment
After QPQ salt bath nitriding technology treatment, different process parameters directly affect the permeability of the workpiece, different layer structure on the corrosion resistance is also different, after the oxidation process, the workpiece surface is also increased oxide film. Therefore, a set of standard orthogonal test schemes are designed, as shown in Table 5.4.
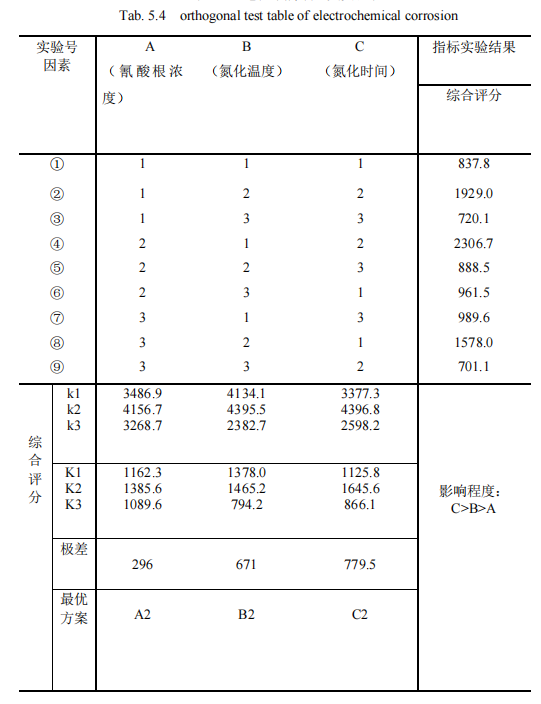
Taking the electrochemical comprehensive score as the index, the orthogonal table is listed, as shown in Table 5.4. In the orthogonal test, when three different factors and three different levels are set, the comprehensive score of the corrosion resistance of the permeating layer changes significantly. The range of the three factors is given in the table as 296, 671 and 779.5, respectively. Obviously, it can be concluded that the range value 779.5 of the nitriding time in the third column is the largest, which indicates that the change of the nitriding time level has the greatest influence on the surface corrosion index. Therefore, the factor of nitriding time is the main factor to be considered in this paper, and the experimental index is the comprehensive score of materials, the greater the value, the better. And the largest of its three levels K1, K2 and K3 is K2=1645.6, so it is best to take its second level. Secondly, in the second column, the range of nitriding temperature 671 is large, which indicates that the change of nitriding temperature level has a great influence on the comprehensive score index of materials. Therefore, the nitriding temperature is the second important factor to be considered in this paper, and the largest of its three levels K1, K2 and K3 is K2=1465.2, so the second level is the best. Finally, it is analyzed that the concentration range of cyanate in the first column is 296, which indicates that the change of cyanate concentration level has the least influence on the experimental index. Moreover, among the three levels K1, K2 and K3, the largest is K2=1385.6, so the second level is the best. Because there is little difference between K2 and K3, combined with the results of actual production and other experimental index groups, comprehensive analysis shows that the influence degree of the three factors is C>. B> A, the nitriding time > Nitriding temperature > Cyanate concentration. The optimal scheme was A2B2C2, that is, 30% cyanate concentration, 570℃ nitriding temperature and 120min nitriding time were the best.
2.2 Surface friction and wear experiment of 42CrMo steel
Surface wear test results of 42CrMo steel
Figure 5.5 and Figure 5.6 show the wear resistance test results of salt bath nitriding specimen and substrate.
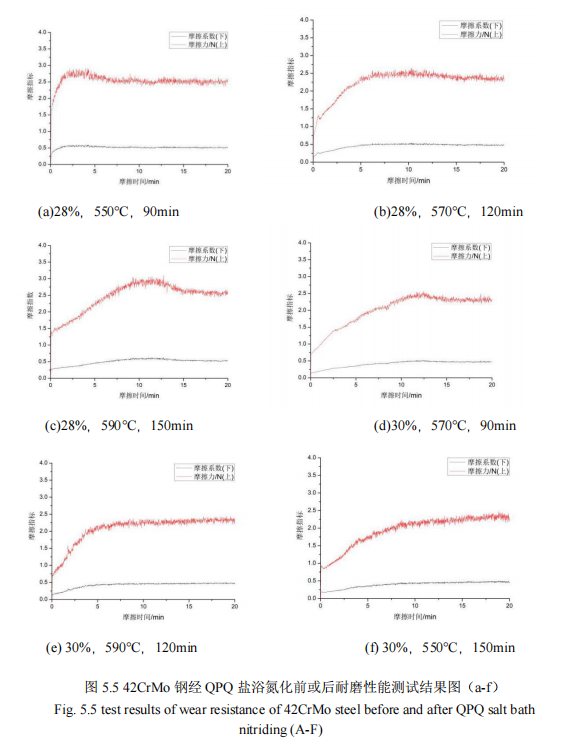
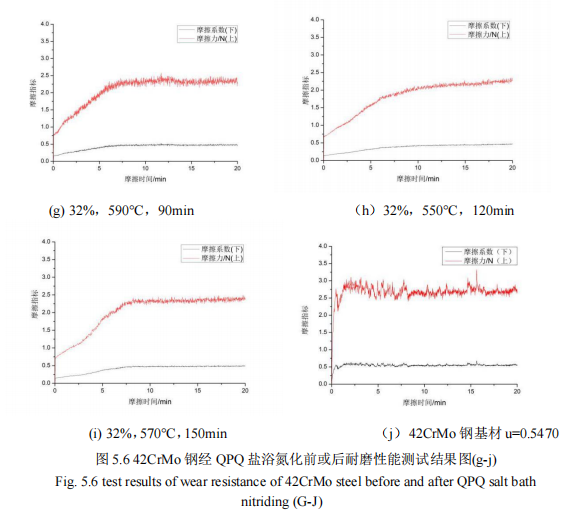
As can be seen from FIG. 5.5 and FIG. 5.6, the upper curve in each figure is the friction force under a pressure of 500N, while the lower curve is the change value of the friction coefficient. After 20 minutes of wear measurement under the same working pressure, the friction coefficient of the nine groups of specimens finally gradually becomes stable. 42CrMo steel after QPQ salt bath nitriding has a different friction coefficient, and the friction coefficient value of the substrate instant from 0 to 0.5 near, and after QPQ salt bath nitriding specimen friction coefficient value is gradually slowly from 0 to 0.5 near, the figure that the friction coefficient is in turn: 0.5172, 0.4582, 0.4993, 0.4094, 0.4233, 0.3953, 0.4230, 0.3725 and 0.4105. The friction coefficient of 42CrMo steel substrate is 0.5470. It can be found that only the friction coefficient of group (a) is greater than 0.5, just like that of the base material, while that of the other experimental groups is less than 0.5. After salt bath nitriding, the friction coefficient of the test pieces is significantly lower than that of the base material, improving the wear resistance of the workpiece. However, the process parameter is 28%, 550℃. The friction coefficient of 90min is not lower than that of the substrate material, which is due to the low concentration of cyanate, low nitriding temperature and short nitriding time of the specimen during nitriding, resulting in less infiltration of nitrogen atoms and smaller penetration layer thickness of the specimen, so the wear resistance of the specimen is not improved. In general, it is because of the existence of the oxide film produced by the oxidation process of QPQ salt bath nitriding, the piston rod 42CrMo steel material surface has higher wear resistance and corrosion resistance, so that the external corrosive substances will not be in direct contact with the nitriding layer, protect the nitriding layer from corrosion, thus improving the wear resistance of the test piece.
Surface wear microstructure of 42CrMo steel
FIG. 5.7 and FIG. 5.8 show the wear morphology of salt bath nitriding specimen obtained by ultra depth of field experiment.
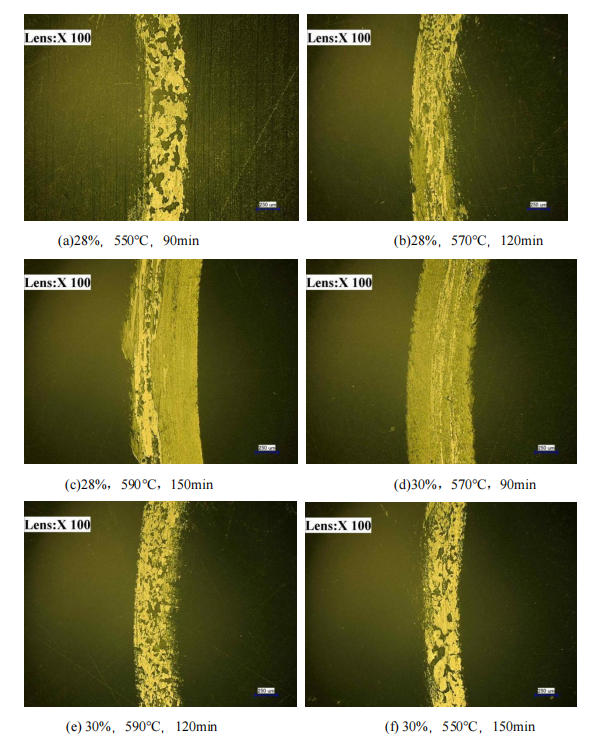
Figure 5.7 wear morphology of 42CrMo steel after QPQ salt bath (A-F)
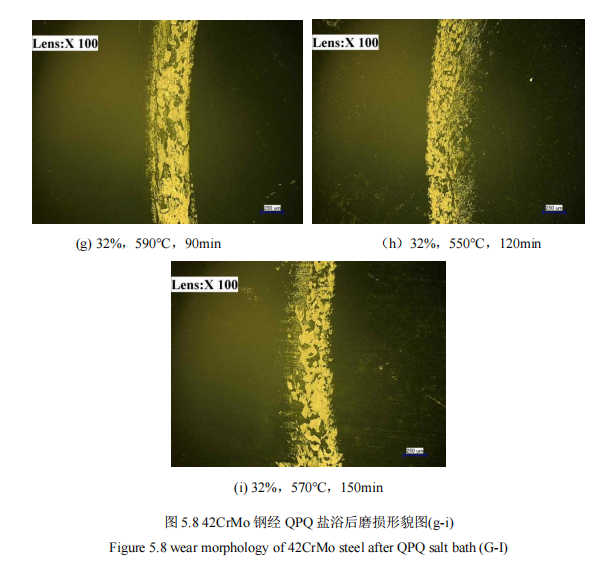
After the test specimen was lowered 100X by the ultra-depth of field instrument, the morphologies of 42CrMo steel after salt bath nitriding were observed after wear, as shown in Figure 5.7 and Figure 5.8. The wear of the first four groups was the most serious, while the wear of the last five groups was relatively light, including 32%, 550℃, 120min and 32%, respectively. 570℃, 150min wear condition is the lightest. However, by observing that the wear condition of the substrate material is the most serious, it can be seen that the wear resistance of the specimen can be greatly provided after the salt bath nitriding of QPQ, and the wear resistance of the specimen can be proved by specific experimental data.
Orthogonal analysis of friction and wear experiment on 42CrMo Steel surface
Nine groups of 42CrMo steel specimens after QPQ salt bath nitriding, respectively do friction and wear test, alcohol cleaning, drying, and weighing the average wear on the scale, design a set of standard orthogonal test scheme, as shown in Table 5.5.
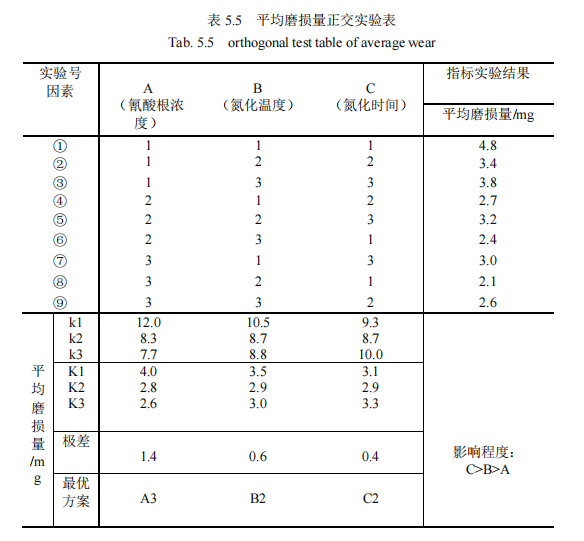
The average wear amount was used as the index to establish the orthogonal experimental group, and the specific situation was shown in Table 5.5. K value was calculated respectively. Finally, the range of the three factors was given in the table as 1.4, 0.6 and 0.4 respectively, and it was obvious that the range of 0.4 of the nitriding time in the third column was the minimum, indicating that among the process parameters, The change of nitriding time has the greatest influence on the experimental index, so the nitriding time is the main factor to be considered in this paper. The experimental index is the comprehensive score, and the smaller the value, the better. In addition, among the three levels K1, K2 and K3, the smallest is K2=2.9, so the second level is the best. Secondly, the range value of 0.6 of nitriding temperature in the second column is small, which indicates that the change of nitriding temperature level has a good influence on experimental indexes. Therefore, the factor of nitriding temperature is the second important factor to be considered in this paper, and the smallest of its three levels K1, K2 and K3 is K2=2.9, so the second level is the best. Finally, it is analyzed that the range value 1.4 of cyanate concentration in the first column is the largest, which indicates that the change of cyanate concentration level has the worst influence on the experimental index. Moreover, among the three levels K1, K2 and K3, the minimum is K3=2.6, so the third level is the best. Based on the above analysis, the influence degree of the three factors is C>. B> A, the nitriding time > Nitriding temperature > Cyanate concentration. The optimal solution was A3B2C2, i.e., 32% cyanate concentration, 570℃ nitriding temperature and 120min nitriding time.
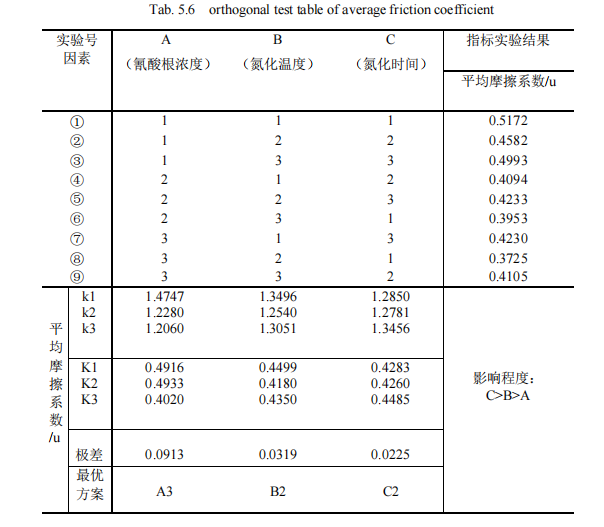
The average friction coefficient was used as the index to establish the orthogonal experimental group, and the specific situation was shown in Table 5.6. K value was calculated respectively. Then, the range of the three factors was given in the orthogonal table as 0.0913, 0.0319 and 0.0225 respectively. This indicates that among the process parameters, the change of nitriding time has the greatest influence on the experimental index, so the factor of nitriding time is the main factor to be considered in the paper, while the experimental index is the comprehensive score, the smaller the value, the better, and among the three levels K1, K2 and K3, the smallest is K2=0.4260, so the second level is the best. Secondly, the range of the nitriding temperature in the second column, 0.0319, is small, which indicates that the change of the nitriding temperature level has a good influence on the experimental indexes. Therefore, the nitriding temperature is the second important factor to be considered in this paper, and the smallest of the three levels K1, K2 and K3 is K2=0.4180, so the second level is the best. Finally, it is analyzed that the concentration range of cyanate in the first column is 0.0913, which indicates that the change of cyanate concentration level has the worst influence on the experimental index. Moreover, among the three levels K1, K2 and K3, the smallest is K3=0.4020, so the third level is the best. Based on the above analysis, the influence degree of the three factors is C>. B> A, the nitriding time > Nitriding temperature > Cyanate concentration. The optimal solution was A3B2C2, i.e., 32% cyanate concentration, 570℃ nitriding temperature and 120min nitriding time.
Step 3: Conclusion
42CrMo steel after QPQ salt bath nitriding treatment, compared with the matrix material, the hardness value increased from 270HV to 800HV, compared with the matrix material increased nearly 3 times, friction coefficient reduced about 0.1, the slope of the Taffir increased 2~4 times, The corrosion current density is reduced by 2~3 orders of magnitude, which greatly improves the wear and corrosion resistance, solves the problem that the mold vibration cylinder is often replaced due to corrosion wear in continuous casting production, greatly improves the work efficiency and reduces the operation cost of production. The conclusion of this paper is expressed in the following aspects.
(1) Under the experimental conditions of cyanate concentration (28 ~ 32) %, nitriding temperature (550 ~ 590) ℃, nitriding time (90 ~ 150) min, oxidation temperature (360 ~ 380) ℃ and oxidation time (15 ~ 25) min, QPQ salt bath nitriding treatment was carried out on 42CrMo steel specimen. The conclusion is that cyanate concentration, nitriding temperature and nitriding time have great influence on the permeability layer thickness and surface hardness of 42CrMo steel specimen.
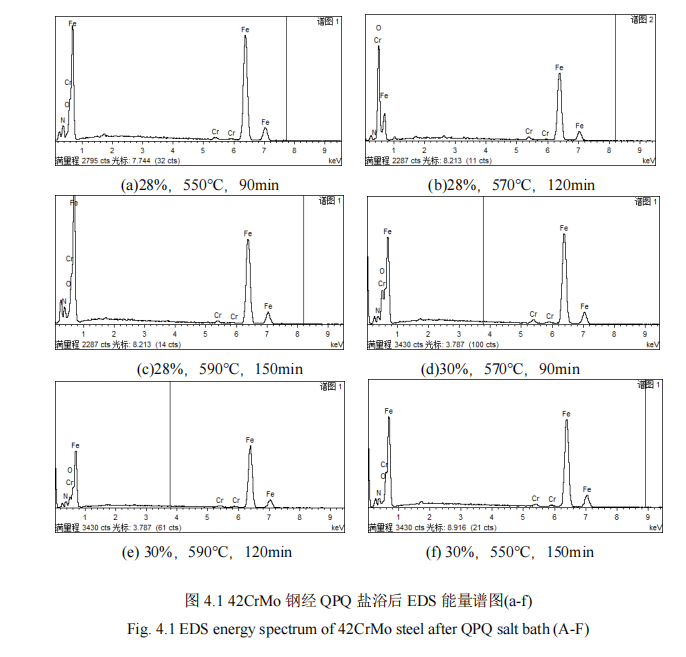
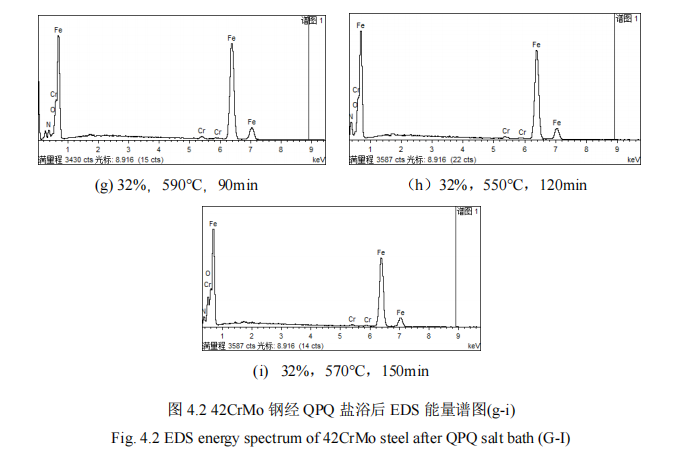
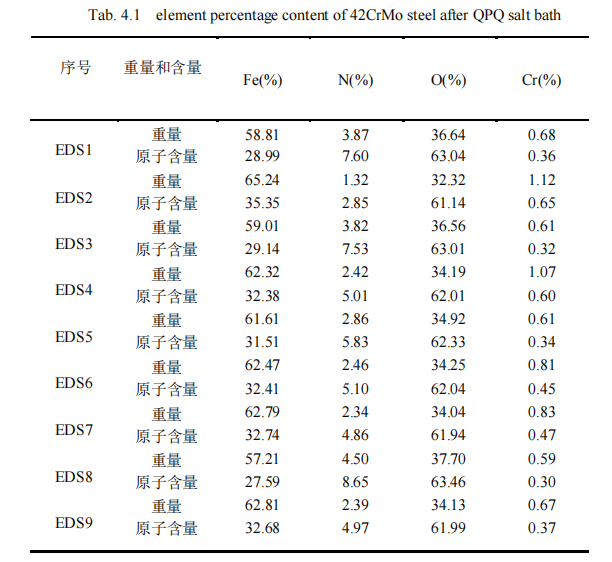
(2) after QPQ salt bath nitriding process after nitriding piston rod 42CrMo steel, infiltration layer is mainly composed of Fe3O4, Fe2N, Fe3N, Fe4N and α-Fe, these new phase refined grain, improve the surface hardness of the material, wear resistance and corrosion resistance; The permeability layer of the specimen presents a layered structure, and a passivated Fe3O4 layer is covered outside the iron nitride layer, which greatly prolonging the corrosion resistance time of the material and improving the wear and corrosion resistance of the material.
(3) The penetration layer thickness, surface hardness, electrochemical corrosion comprehensive score, average wear and friction coefficient as indicators, with cyanate concentration, nitriding temperature, nitriding time as factors to do orthogonal analysis, the optimal QPQ salt bath nitriding process parameters of 42CrMo steel piston rod A3B2C2, The concentration of cyanate, nitriding temperature and nitriding time were 32%, 570℃ and 120min, respectively. The influence degree of the optimal factor is C> B> A, the nitriding time > Nitriding temperature > Cyanate concentration.

Introduction And Classification Of Annealing Process Gas Nitriding Heat Treatment Process Heat Treatment Of Nanocrystalline Core


Contact us
Your email address will not be published. Required fields are marked *