Study on QPQ Treatment Technology of 05Cr17Ni4Cu4Nb Material Surface
In order to improve the wear resistance of 05Cr17Ni4Cu4Nb material, extend its service life, and expand its application range, the surface of 05Cr17Ni4Cu4Nb stainless steel material is strengthened by QPQ heat treatment process. The effects of different nitriding time and concentration of cyanate ions in base salts on the thickness and hardness of the surface penetration layer were studied by scanning electron microscopy and microhardness tester. The results show that for 05Cr17Ni4Cu4Nb stainless steel bar, the surface penetration layer thickness can reach 20 ~ 30μm when the concentration of cyanate ion is 32% ~ 35%, 35% ~ 38%, and the nitriding time is 30 ~ 40 min and 35 ~ 45 min, respectively. For 05Cr17Ni4Cu4Nb stainless steel sheet, when the concentration of cyanate ion is 32% ~ 38% and the nitriding time is 30 ~ 40 min, the surface penetration layer thickness can reach 20 ~ 30 μm. The optimized heat treatment process of QPQ has certain guiding effect on the practical application of 05Cr17Ni4Cu4Nb stainless steel.
05Cr17Ni4Cu4Nb steel is a precipitation-hardened stainless steel prepared by adding Cu, Nb and other strengthening elements on the basis of Cr17 stainless steel. Due to its low carbon content (C content ≤ 0.08%), high chromium and copper content, the steel has good corrosion resistance and is often used as parts requiring both weak acid, alkali and salt corrosion resistance and high strength [1,2]. The solid solution treatment temperature range of this material is 1 020 ~ 1 060 ℃, in which the solid solution treatment at 1 040 ℃ for 60 min can obtain relatively excellent seawater corrosion resistance and good processing performance [3]. After processing into parts, it is generally placed in 450 ~ 580℃ conditions, heat preservation for 4 h, after aging treatment, can get good comprehensive performance. However, when the material is used in some working conditions requiring long-term wear or long service life, it is restricted by its lack of wear resistance.
By using the composite process composed of nitriding and oxidation process — QPQ(Quench-Polish-Quench) salt bath composite treatment technology, can form a high quality penetration layer with enough depth and certain hardness on the surface of parts, improve the surface hardness of parts at the same time, The surface of the parts has strong wear resistance, corrosion resistance, fatigue resistance and anti-bite ability. This technology is often used to replace heat treatment and surface strengthening technologies such as carburizing, ion nitriding and chromium plating [4,5]. With relatively low cost, this technology has been applied to improve the wear resistance, fatigue strength and corrosion resistance of tools, molds and auto parts [6,7]. In this work, 05Cr17Ni4Cu4Nb stainless steel material as the base material, according to the actual engineering application requirements, the use of QPQ treatment process on its surface wear-resistant strengthening treatment, focusing on the different nitrizing time and the concentration of cyanate ions in the base salt on the surface of the material penetration layer thickness and hardness, in order to obtain the best QPQ treatment process. Improve the wear resistance of the surface of the material parts, prolong the service life of the parts, and provide the basis for the popularization and application of the material.

1. Experiment
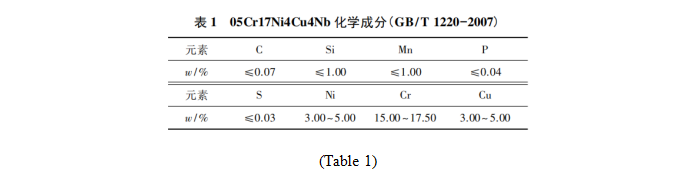
05Cr17Ni4Cu4Nb stainless steel was selected as the test material. The material was in solid solution state. The chemical composition was shown in Table 1. The bar material with a diameter of 60 mm is processed into a sample with a thickness of about 1 mm. The plate with a thickness of 1 mm was processed into a sample with a diameter of about 60mm, and the bar and plate samples were tested respectively. First, all samples were aged at 480 ℃ for 4 h, and the hardness of bar samples reached HRC 42.5 ~ 44.0, and that of sheet samples reached HRC 35.0 ~ 42.0. In order to obtain better surface roughness after QPQ treatment and lay a foundation for subsequent surface finishing process, all samples were pre-polished for 30min by using water sand and rolling liquid in VA36 /I vortex grinding machine.
QPQ treatment process: ① The surface of the sample with metal cleaning agent after oil removal decontamination, rinse clean water; ② In the air furnace 350 ~ 400 ℃, hold for 20 ~ 40 min for preheating treatment; ③ Salt bath nitriding formed nitriding layer and diffusion layer; ④ salt bath oxidation form oxide film; (5) The residual oxide salts on the surface of the sample were dissolved in cold water, and then rinsed in hot water at 70 ~ 80 ℃ for 10 min. ⑥ Surface dry; ⑦ Protection by immersion anti-rust oil. After QPQ treatment, the infiltration layer structure formed on the surface of the parts is composed of three layers: from the outside and inside are oxide film, nitriding layer and diffusion layer. Oxide film is the oxide of iron, can improve the corrosion resistance of the metal surface, beautify the appearance of the workpiece, and the nitriding layer together to form a very high corrosion resistance of the comprehensive corrosion resistant layer. Nitriding layer, commonly known as white bright layer, is the most important part of the permeating layer organization formed by QPQ technology. Its wear resistance and corrosion resistance are extremely high. The quality of the permeating layer plays a vital role in improving the surface hardness and wear resistance of parts. The diffusion layer is a dark colored structure between the nitride layer and the material substrate, which is the solid solution or supersaturated solid solution of nitrogen in iron. Generally, the main function of diffusion layer is to improve the fatigue strength of metal [8,9]. The quality of nitriding layer has an important relationship with nitriding temperature, nitriding time and cyanate ion concentration in base salt during QPQ treatment. The nitriding temperature is controlled within 520 ~ 580 ℃ according to the actual demand. The concentration of cyanate ion is generally controlled within the range of 32% ~ 38%. When the concentration is lower than 32%, the penetration rate is slow and the surface hardness is low. When the salt bath is higher than 38%, the oxidation of salt bath is enhanced, which is easy to cause the permeability layer is not dense, or even seriously loose [4,10]. In the process of QPQ treatment, the base salt developed by Chengdu Tool Research Institute was used for 30 min preheating treatment at (380±3) ℃, the temperature of nitriding treatment was controlled at (570±3) ℃, the temperature of oxidation treatment was controlled at (400±3)℃, and the time was controlled at 15 min. The optimal process parameters were obtained by controlling different cyanate ion concentration and nitriding time.
2. Results and analysis
During the test, the concentration of cyanate ions in base salt was divided into low concentration (32%-35%, mass fraction, the same below) and high concentration (35%-38%). One group of tests were completed in the low concentration area and the high concentration area respectively, with 8 plate and bar samples in each group. Two samples were taken out at four points at nitriding time of 30, 40, 50 and 70 min respectively. The surface hardness and penetration layer thickness of the samples were tested by HVS-1000A digital microhardness tester and VEGAIIXMU scanning electron microscope. The results are shown in Table 2.
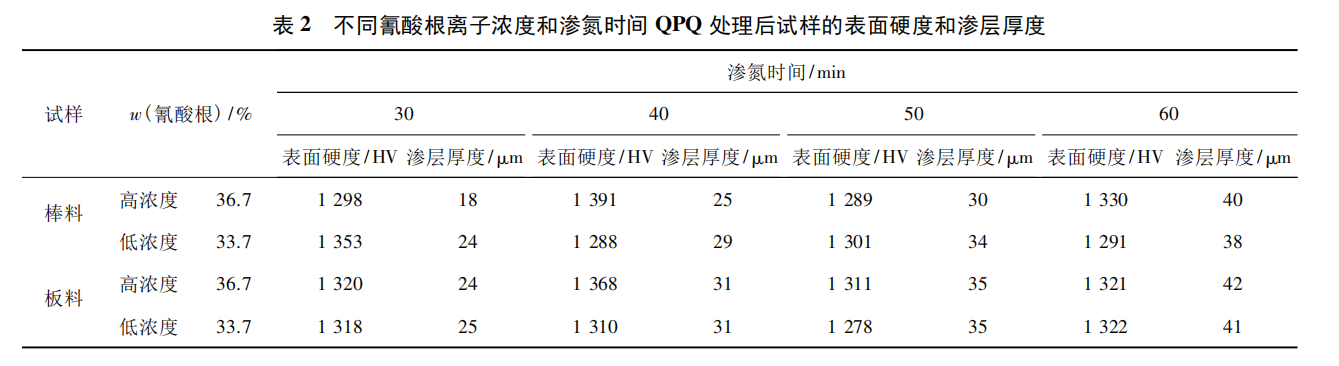
It can be seen from Table 2 that under all test parameters, the surface microhardness of the samples can reach more than 1 200 HV. When the concentration of cyanate ion is controlled in the range of 32% ~ 38%, no matter in high concentration or low concentration area, the influence on the hardness of the sample surface is small. Under the condition of constant cyanate ion concentration, the thickness of the nitriding layer has a linear relationship with the nitriding time. However, with the extension of the nitriding time, the surface hardness of the sample tends to decrease. This is because the outer surface of the nitriding layer of the sample becomes more and more relaxed with the extension of the nitriding time, so it is necessary to control the nitriding time within a reasonable range.
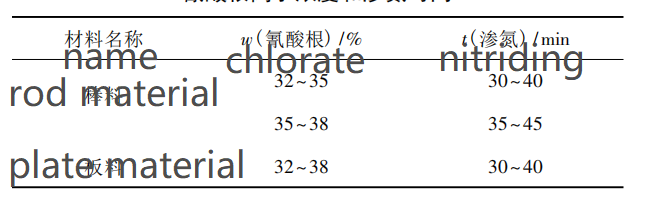
Under normal circumstances, the surface roughness value of the parts after QPQ treatment will rise slightly. In order to obtain better appearance quality, the above samples after QPQ treatment are polished. The results show that, compared with other samples, the surface of the nitriding layer thickness of more than 30 μm is more prone to collapse phenomenon (that is, small area of nitriding layer shedding phenomenon). This is due to the high nitride content and hardness in the nitriding layer. Too thick nitriding layer will lead to increased brittleness on the surface of the parts, and then lead to the collapse phenomenon in the finishing process. Therefore, it is suggested that the permeability layer thickness of 05Cr17Ni4Cu4Nb stainless steel parts should be controlled within the range of 20 ~ 30 μm during QPQ treatment. The cyanate ion concentration and nitrizing treatment time of rod and plate recommended by QPQ treatment according to the permeability layer thickness are shown in Table 3.
Table 3 cyanate ion concentration and nitriding time of 05Cr17Ni4Cu4Nb samples treated with QPQ
3. Conclusion:
By studying the QPQ treatment process of 05Cr17Ni4Cu4Nb material surface, more reasonable process parameters are obtained: For the bar material, when the concentration of cyanate ion is 32% ~ 35%, 35% ~ 38%, and the nitriding time is 30 ~ 40 min, 35 ~ 45 min, the surface penetration layer thickness can reach 20 ~ 30 μm. When the concentration of cyanate ion is 32% ~ 38% and the nitriding time is 30 ~ 40 min, the surface penetration thickness of the material can reach 20 ~ 30 μm, which has a certain guiding effect on the surface QPQ treatment of this material and similar materials. It is suggested that before QPQ treatment of parts, the sample should be pretested first, and the optimal cyanate ion concentration and nitriding time should be determined through the pretest before formal treatment.

Improvement of Carburizing and Quenching Taper Deformation of Driven Gear Study on Softening Annealing Process of Low Carbon Steel Wire QT40-10 Ductile Iron Heat Treatment Process


Contact us
Your email address will not be published. Required fields are marked *