Effect of Annealing Process on Structure, Magnetic Properties and Brittleness of Fe81Si4B12Cu1P2 Amorphous Alloy Ribbons
1. Research progress on annealing process of nanocrystalline soft magnetic alloys
Annealing the amorphous soft magnetic alloy can not only eliminate the residual internal stress generated during the rapid solidification process, reduce the stress-induced anisotropy, but also obtain nano-sized grains and form an amorphous-nanocrystalline dual-phase structure. The formation of this structure greatly reduces the magnetocrystalline anisotropy and saturation magnetostriction coefficient [62], resulting in materials with excellent soft magnetic properties such as low coercive force and high initial magnetic permeability. The microstructure and physical properties of amorphous nanocrystals are closely related to the heat treatment parameters during crystallization annealing. Studies have shown that the heating rate, annealing temperature, holding time, atmosphere and cooling methods in annealing will affect the grain size and volume fraction of nanocrystals [27], thereby affecting the magnetic properties of the final amorphous nanocrystalline alloy. The annealing method has also been greatly developed. In addition to isothermal annealing, step annealing, rapid annealing, and Joule annealing have been developed in recent years.
2. Effect of annealing process on structure, magnetic properties and brittleness
Fe81Si4B12Cu1P2 alloy strips with completely amorphous structure were obtained by single-roll rapid quenching equipment. This chapter studies the effect of isothermal annealing process on the structure and properties of Fe81Si4B12Cu1P2 alloy. The process curve is shown in Figure 5.1. The purpose of the annealing treatment is to change the fabricated thin ribbons into nanocrystals.
Isothermal annealing temperature is an important process parameter. Different selection of annealing temperature can form alloys with different grain size, crystallization volume fraction and lattice constant, which in turn affects the soft magnetic properties of the material. The determination of the annealing temperature of an amorphous alloy is determined by its crystallization temperature, and the crystallization temperature can be measured by differential scanning calorimetry. Figure 5.2 is the DSC curve measured at a heating rate of 25K/min for the quenched Fe81Si4B12Cu1P2 amorphous alloy. There are two crystallization exothermic peaks on the DSC curves, and they are far apart. The first crystallization peak corresponds to the precipitation of the α-Fe(Si) phase, and the second crystallization peak is the crystallization of the remaining amorphous phase, which is mainly related to the precipitation of Fe-B and Fe-P compounds. The initial crystallization temperatures of the two crystallization peaks are Tx1≈716K and Tx2≈822K, respectively. From this we determined that the heat treatment temperature range is between 623~873K. The annealing time is set at 1~10min, and the annealing heating rate is set at 0.05~5K/s.
Use a rapid annealing furnace for annealing. During annealing, put the sample into a quartz tray, pass argon for about 20 minutes, turn on the power to heat after the air in the tube is cleaned, and when the furnace temperature rises to the set temperature, put the sample into the insulation state, after the heat preservation is over, the heating power is automatically disconnected, and the sample is cooled to room temperature with the furnace under a high-purity argon atmosphere.

3. Effect of annealing process on structure
It is the XRD pattern of amorphous alloy Fe81Si4B12Cu1P2 after heat treatment at different annealing temperatures, where the annealing time and annealing rate are 3min and 0.5K/s, respectively. It can be seen from the figure that after annealing at 623K, the X-ray pattern consists of a diffuse packet, indicating that the alloy is still amorphous, while after annealing between 723 and 873K, the XRD pattern shows sharp crystallization at 45° peak, indicating that the alloy begins to form a crystalline phase in this temperature range, and this sharp peak corresponds to the formation of α-Fe(Si) nanocrystalline phase. It can also be seen from the XRD spectrum that when the annealing temperature is higher than 723K, the intensity of the diffraction peak gradually increases with the increase of the annealing temperature Ta, indicating that the amount of α-Fe(Si) phase precipitation increases with the increase of Ta .
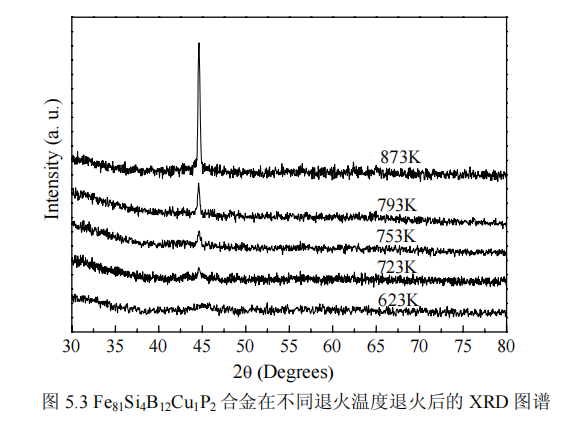
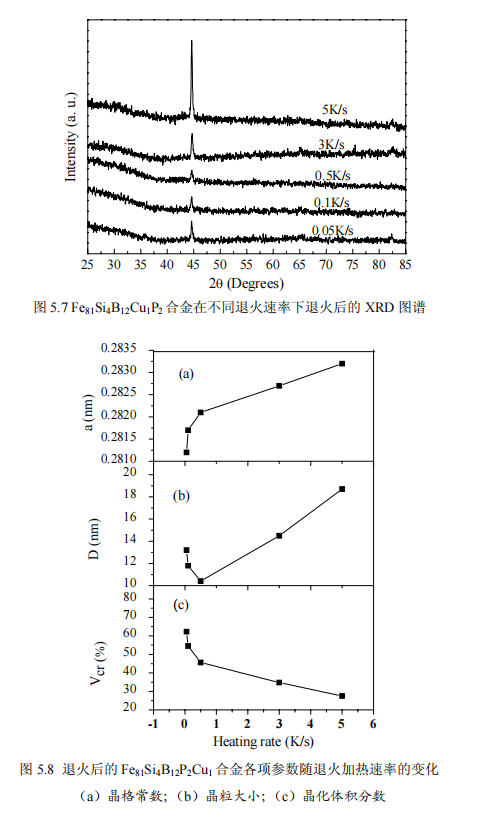
By analyzing the XRD pattern, the lattice constant a can be obtained, as shown in Figure 5.4a, the lattice constant is between 0.2793 and 0.2824nm, and it can be seen that the lattice constants are all smaller than the lattice constant of Fe (0.286nm). This is because a small amount of Si atoms replace Fe atoms in the nanocrystals, forming the Fe3Si phase of DO3 structure. As the annealing temperature increases, the lattice constant decreases from 0.2824 to 0.2793nm, which indicates that increasing the annealing temperature increases the dissolution of Si in the α-Fe(Si) phase, which may be due to the fact that the increase in temperature is conducive to the diffusion of Si atoms into the α-Fe(Si) nanocrystalline phase. The relationship between the average grain size of α-Fe(Si) and the annealing temperature is shown in Fig. 5.4b. The average grain size D is calculated using the half-width of the X-ray diffraction peak using the Scherrer formula (2-2). The average grain size of α-Fe(Si) nanocrystalline phase increases from 9.8nm to 21.8nm, that is, the grain size increases sharply with the increase of annealing temperature. Figure 5.4c shows the relationship between the nanocrystal fraction and the annealing temperature, and the nanocrystal fraction is obtained according to (3-1). It can be seen that the fraction of nanocrystals increases from 32.7% to 68.3%, that is, as the annealing temperature increases, so does the fraction of nanocrystals. During the annealing process, due to the repulsion and attraction between Fe and Cu and Cu and P [56–58], some Cu and P-rich regions are formed in the FeSiBCuP amorphous alloy, and the number of this region reaches a certain density , it will be evenly distributed in the amorphous matrix, providing a site for the nucleation of α-Fe(Si) and refining the grains. In addition, Cu-P clusters can act as diffusion barriers to prevent the growth of α-Fe(Si) grains. The average grain size and fraction of nanocrystals are related to the density of Cu-P rich clusters. With the increase of annealing temperature, due to the increase of Cu-P clusters, that is, the increase of nucleation sites provided, the grain size decreases and the integral fraction of nanocrystals increases; however, with the further increase of annealing temperature, although Cu-P clusters As clusters increase, the integral fraction of nanocrystals increases further, but the grains begin to grow, resulting in an increase in the average grain size.
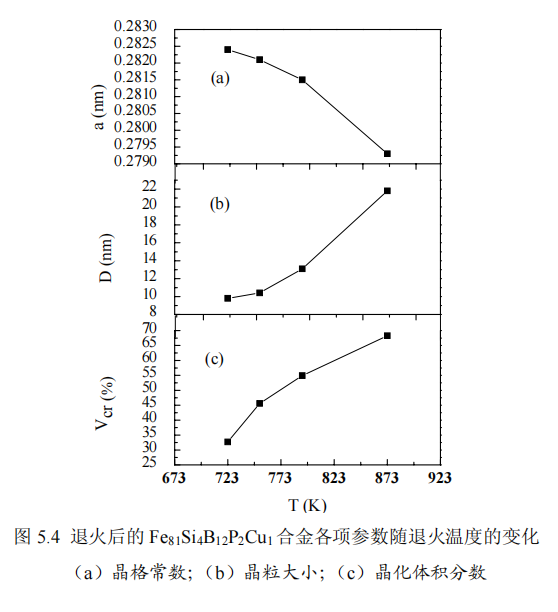
Influence of holding time on structure
XRD pattern of amorphous alloy Fe81Si4B12Cu1P2 annealed at 753K with different holding time t, where the annealing heating rate is 0.5K/s. It can be seen from the figure that a sharp peak appears in the XRD pattern at 45°, indicating that the entire alloy begins to crystallize, and this sharp peak corresponds to the formation of α-Fe(Si) nanocrystalline phase. At the same time, as the holding time t increases, the intensity of the diffraction peaks gradually increases, indicating that the amount of α-Fe(Si) phase precipitation increases with the increase of t. By analyzing the XRD pattern, the lattice constant a can be obtained, as shown in Figure 5.6a, the lattice constant is between 0.2807 and 0.2823nm, and it can be seen that the lattice constants are all smaller than the lattice constant of Fe (0.286nm). As mentioned earlier, a small amount of Si atoms replaced Fe atoms in nanocrystals to form Fe3Si phase with DO3 structure. With the increase of holding time, the lattice constant decreased from 0.2823nm to 0.2807nm, which indicated that increasing holding time The dissolution of Si in the α-Fe(Si) phase increases, which may be due to the fact that the prolongation of time is conducive to the diffusion of Si atoms into the α-Fe(Si) nanocrystalline phase. Figures 5.6b and 5.6c show the relationship between the average grain size and nanocrystal fraction of α-Fe(Si) and the holding time, respectively. The average grain size and nanocrystal fraction are according to Scherrer formula (2-2) and Obtained by formula (3-1), it can be seen that the average grain size increases from 10.1nm to 15.3nm, and the integral fraction of nanocrystals increases from 38.2% to 56.7%. The average grain size and fraction of nanocrystals are related to the density of Cu-P rich clusters. In the case of constant annealing temperature, as the holding time prolongs, the Cu-P clusters will continue to increase, that is, the nucleation sites provided will increase, and the formed nanocrystals will continue to grow, so the average grain size of the alloy and The integral fraction of nanocrystals increases with the increase of holding time t.
Effect of Annealing Heating Rate on Structure
Figure 5.7 shows the XRD patterns of amorphous alloy Fe81Si4B12Cu1P2 at five different annealing heating rates. The annealing temperature is 753K and the holding time is 3min. It can be seen from the figure that a sharp peak appears in the X-ray diffraction pattern at 45°, indicating that the entire alloy begins to crystallize, and this sharp peak corresponds to the formation of α-Fe(Si) nanocrystalline phase. By analyzing the XRD pattern, the lattice constant a can be obtained, as shown in Figure 5.8a, the lattice constant is between 0.2812 and 0.2834nm, and it can be seen that the lattice constants are all smaller than the lattice constant of Fe (0.286nm). As mentioned earlier, a small amount of Si atoms replaced Fe atoms in the nanocrystals, forming the Fe3Si phase of DO3 structure. As the annealing rate increases, the crystal constant increases from 0.2812 nm to 0.2834, which indicates that the dissolution of Si in the α-Fe(Si) phase decreases with increasing annealing rate, which may be due to the decrease of Si diffusion into Time in the α-Fe(Si) nanocrystalline phase. Figures 5.8b and 5.8c show the relationship between the average grain size and nanocrystal fraction of α-Fe(Si) and the annealing heating rate. The average grain size and nanocrystal fraction are according to Scherrer formula (2-2) and It can be seen that the average grain size decreases first and then increases, while the integral fraction of nanocrystals decreases sharply. This is because in the case of constant annealing temperature and holding time, as the annealing heating rate increases, the average grain size decreases initially due to the shortening of the time required for grain growth; however, as the annealing rate further increases Cu and P elements have no time to diffuse, resulting in a decrease in the density of Cu-P clusters, that is, a decrease in the nucleation sites provided, an increase in the grain size, and a decrease in the integral fraction of nanocrystals.
4. Effect of annealing process on magnetic properties
The magnetic properties of Fe81Si4B12Cu1P2 alloy after annealing at different temperatures were measured, the holding time was 3min, and the annealing rate was 0.5K/s. Figure 5.9 shows the coercivity and saturation magnetization curves of the Fe81Si4B12Cu1P2 quenching alloy thin strip with the annealing temperature. It can be clearly seen from the figure that the coercive force decreases with the increase of the annealing temperature when the annealing temperature Ta is less than 753K, the coercive force increases sharply when the annealing temperature Ta is greater than 753K, and the coercive force reaches the minimum at 753K. It shows that increasing the annealing temperature is beneficial to improve the magnetic properties of the alloy, but the higher the annealing temperature is not the better, because at high temperature, the grain grows sharply, and the increase of the grain will directly affect the size of the coercive force, resulting in the occurrence of alloy A phenomenon in which the magnetic properties decrease. However, if the annealing temperature is too low, the crystallization is not complete, which is not conducive to the formation of a uniform amorphous nanocrystalline structure, and the coercive force and saturation magnetization are very low. According to the formula (3-2), it can be seen that the increase of the volume fraction of nanocrystalline phase is conducive to the increase of saturation magnetization. As the temperature increases, the volume fraction of nanocrystalline phase increases, so the saturation magnetization increases with the increase of annealing temperature.

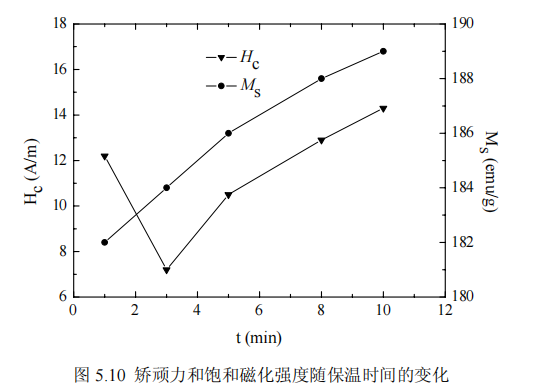
The influence of holding time on magnetic properties The magnetic properties of Fe81Si4B12Cu1P2 alloy after different annealing and holding times were measured, the annealing temperature was 753K, and the annealing heating rate was 0.5K/s. Figure 5.10 shows the coercive force and saturation magnetic induction of the alloy as a function of annealing holding time after annealing at 753K. It can be seen that when the holding time is extended from 1 min to 3 min, the slope of the coercive force curve decreases greatly, and the coercive force decreases with the extension of the holding time. The coercive force of the alloy is the minimum when the annealing holding time is 3min. When the holding time is extended from 3min to 10min, the coercive force increases monotonously, indicating that extending the holding time is beneficial to improve the magnetic properties of the alloy, but it is not that the longer the holding time, the better, because at a certain temperature, the holding time is long, and the grains are sharp The growth of the grains will directly affect the size of the coercive force, resulting in a decrease in the magnetic properties of the alloy. If the annealing time is too short, the crystallization is incomplete, and the coercive force and saturation magnetization are very low. If the annealing time is too long, the grains will be coarsened, the exchange coupling effect will be reduced, and the magnetic properties will be reduced. Under the condition that other experimental parameters remain unchanged, appropriate holding time is conducive to the formation of uniform amorphous nanocrystalline structure and the elimination of lattice defects, which is an important reason for the improvement of magnetic properties [97]. With the increase of holding time, the volume fraction of nanocrystalline phase increases. According to (3-2), it can be concluded that the saturation magnetization increases with the increase of annealing holding time.
Effect of Annealing Heating Rate on Magnetic Properties
The magnetic properties of Fe81Si4B12Cu1P2 alloy after annealing at different annealing heating rates were measured, the annealing temperature was 753K, and the holding time was 3min. Figure 5.11 shows the curve of the magnetic properties of the alloy as a function of the annealing heating rate. It can be seen that the coercive force of the alloy Fe81Si4B12Cu1P2 shows a trend of decreasing first and then increasing, and the coercive force is the smallest when the annealing rate is 0.5K/s , is 7.2A/m, indicating that the increase of the annealing rate is conducive to improving the magnetic properties of the alloy, but the faster the annealing rate, the better, because the alloy crystallization is not complete if the annealing rate is too fast, and the coercive force and saturation magnetization are very low. Low. When the annealing rate is slow, the crystal grains have sufficient time to grow, and the growth of the crystal grains will directly affect the size of the coercive force, resulting in a decrease in the magnetic properties of the alloy. The saturation magnetization and annealing rate of the sample decrease almost monotonously, because as the annealing rate increases, the volume fraction of the nanocrystalline phase decreases. According to the formula (3-2), it can be concluded that the saturation magnetic induction increases with the increase of the annealing rate reduce.
Comprehensive Analysis of Effect of Annealing Process on Magnetic Properties
A schematic diagram of the effect of the annealing process on the phase composition and microstructure of the alloy is shown in Figure 5.12. When the crystallization temperature is low, the crystallization time is short, and the annealing heating rate is fast, the crystallization is incomplete, and there are still some amorphous phases or intermediate metastable phases, and the grain distribution is uneven (as shown in Figure 5.12a), which affects the exchange rate. Coupling. However, if the crystallization temperature is too high, due to the rapid growth of the α-Fe(Si) phase at high temperature, the grains will be coarse and uneven (as shown in Figure 5.12c); if the crystallization time is too long, the grains will grow sufficiently, and the Coarse grains will appear (as shown in Figure 5.12c), and the crystallization time should be appropriately shortened, and the crystallization process will be over before the grains grow. The grains can be refined to make the grain size uniform (as shown in Figure 5.12b). Improve the microstructure; if the annealing rate is high, the clusters that provide nucleation sites will not be formed in time, and the grain size will also be coarse (as shown in Figure 5.12c). Therefore, only under appropriate annealing process parameters, the alloy is composed of nanocrystalline and amorphous phases, and the microstructure is fine and uniform, which is conducive to exchange coupling and its magnetic properties are relatively excellent.

Effect of Annealing Process on Brittleness
There are many structural defects inside the amorphous alloy made by the rapid solidification method. During the annealing process, the atoms, molecules and these structural defects in the alloy undergo thermal activation motion, which causes the metastable structure and internal structure of the amorphous alloy. When the defect changes, especially when the density value of the defect changes, its distribution may change from scattered to concentrated, which increases the stress sensitivity and leads to the embrittlement behavior of the amorphous alloy. Moreover, the atomic diffusion behavior during the annealing process makes these defects interact with each other to cause configuration changes, resulting in structural relaxation, which will also lead to embrittlement of amorphous alloys. With the change of annealing process, certain crystallization and phase separation will occur in amorphous alloys, which are also important factors leading to embrittlement of amorphous alloys [98, 99].
Figures 5.13-5.15 show the change of fracture strain εf of amorphous alloy samples under different annealing processes. The third chapter mentions that the amorphous alloy Fe81Si4B12Cu1P2 has good toughness in the quenched state, and the value of the fracture strain εf is 1, but the samples undergo different degrees of embrittlement after different annealing processes, and the influence of different annealing process parameters on embrittlement to varying degrees. Due to some errors in the measurement of brittleness, we can only see the trend of the change of the bending fracture strain value εf after different annealing processes, which provides a certain basis for the evaluation of brittleness.
It can be seen from Figure 5.13 that annealing increases the brittleness of the amorphous strip, which is due to the short-range structural relaxation in the amorphous alloy, resulting in the uneven distribution of elements. But the distribution of brittleness and annealing temperature is not linear. When the temperature is low, the free volume decreases, and the brittleness increases with the increase of the annealing temperature; but when the annealing temperature reaches the crystallization temperature, the brittleness decreases due to the appearance of nanocrystals, and the distribution of the grains is uniform at 753K, and the brittleness is relatively low, indicating that the small Precipitation of size nanocrystalline particles improves the brittleness; as the temperature rises further, the particles grow larger and the brittleness increases, but the brittleness is slightly improved after annealing at 953K, which is related to the precipitation of FeB and FeP crystallization phases in the amorphous.
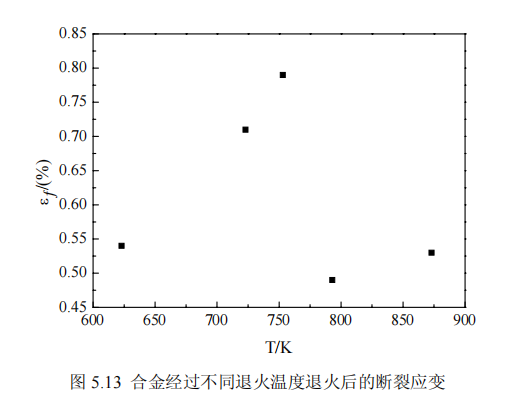
Figure 5.14 shows the effect of annealing soak time on brittleness. The alloy was annealed at 753K, and after holding for 1min, 3min, 5min, 8min and 10min respectively, the brittleness was compared. It was found that with the extension of the holding time, the degree of crystallization increased, and uniformly distributed amorphous and nanocrystalline structures were obtained. The internal metastable structure tends to be stable, and the toughness of the alloy improves. However, the longer the holding time, the better. When the holding time is longer than 3 minutes, the brittleness will increase due to the growth of the grains.

Figure 5.15 shows the effect of annealing heating rate on brittleness: when the annealing heating rate is slow, the nanocrystals have sufficient time to grow up, making the strip texture brittle and losing good toughness, which is not conducive to application; but the annealing rate If it is too fast, the nanocrystals will not have time to nucleate, and a uniform amorphous nanocrystal structure cannot be obtained, and the brittleness will increase accordingly.

The essence of the embrittlement process of the amorphous strip is the release process of the internal stress in the strip. Increasing the temperature, prolonging the holding time and reducing the annealing rate are all conducive to the full diffusion and position adjustment of atoms, but the diffusion of atoms must first meet the thermodynamic conditions That is, the temperature condition, so the degradation temperature plays a decisive role in the embrittlement of the amorphous strip.
In this chapter, the effects of annealing process on the structure, magnetic properties and brittleness of Fe81Si4B12Cu1P2 amorphous alloy strips are studied, and the following conclusions are drawn:
(1) The metastable amorphous alloy will undergo amorphous crystallization after annealing, and it will transform to a stable crystallization phase. The annealing temperature, holding time and annealing heating rate directly affect the different crystallization degrees of the material: holding time No change, the annealing heating rate is the same, the temperature is too low or too high; the temperature is constant, the annealing heating rate is the same, the holding time is too long or too short; Slow is not conducive to full crystallization and control of grain size. The Fe-based amorphous alloy with a certain composition can precipitate a single uniform bcc structure α-Fe(Si) crystal phase on the amorphous substrate after proper annealing treatment, and a multi-phase structure composed of amorphous and nanocrystalline can be obtained.
(2) When the annealing temperature is low, the atomic diffusion is slow, the nucleation sites are few, the crystallization process is stagnant, and a uniform amorphous nanocrystalline structure cannot be formed, the magnetic properties are poor, and the brittleness is enhanced; when the annealing temperature is 753K, due to Form uniform and fine nanocrystals with the smallest coercive force and improved brittleness; as the temperature increases, the grain grows, the coercive force increases, and the brittleness increases; when the temperature continues to rise, FeB, FeP, etc. are precipitated from the residual amorphous Metal compounds, although the magnetic properties are drastically reduced, the brittleness is slightly improved.
(3) When the annealing temperature is constant, the annealing time is short, the nucleation sites are less, and a uniform structure cannot be formed, the magnetic properties are reduced, and the brittleness is increased; the increase of the annealing time, the grain size distribution is uniform, and good magnetic properties and brittleness are obtained. It is improved; the annealing time is further increased, the grains have sufficient time to grow, the magnetic properties are reduced, and the brittleness is increased.
(4) When the annealing heating rate is slow, the grains have sufficient time to grow, the magnetic properties decrease, and the brittleness increases; the increase of the annealing rate inhibits the crystallization transformation of the alloy, inhibits the grain growth of the crystallized phase, and makes the grain The particle size distribution is uniform, good magnetic properties are obtained, and the brittleness is improved; but the annealing rate is further increased, the atoms have no time to diffuse, the nucleation position is reduced, and it is not conducive to the formation of a uniform structure, the magnetic properties are reduced, and the brittleness is increased.

QPQ Salt Bath Liquid Nitriding Process And Quality Control QPQ Nitriding Process Experiment And Heat Treatment Process of 42CrMo Piston Rod Quenching Oil Tank Oil Fume Purification Device

Contact us
Your email address will not be published. Required fields are marked *