QPQ Nitriding Process Experiment And Heat Treatment Process of 42CrMo Piston Rod
1. Experimental materials
QPQ salt bath nitriding process used experimental materials include 42CrMo steel material, salt nitride, salt oxide, salt adjustment, sandpaper (500#, 2000#) and metallographic polishing flannelette.
1.1 Experimental medium material
Experimental media for salt bath nitriding mainly include salt nitride, oxidized salt and adjusted salt, as shown in Figure 2.1.

Nitriding salt: also known as white base salt, is extracted from urea, its internal contains a large number of active nitrogen atoms, through the air flow of high temperature heating, after slowly melting for black nitriding salt bath. In actual production, basic salt is generally added to the position of 3/4 of the crucible, and appropriate amount of basic salt is gradually added after the salt bath surface drops, and appropriate amount of adjusted salt is also required to be added regularly to ensure the stability of cyanate concentration value. Moreover, the concentration of active nitrogen atoms in salt nitride directly affects the permeability thickness of the specimen, as shown in FIG. 2.1(a).
Salt oxide: also known as AB salt, sodium nitrate, sodium carbonate and sodium hydroxide in accordance with A certain proportion of mixed, and salt oxide composition proportion directly affects the corrosion resistance of the workpiece, because the proportion involves the company’s core technical problems, this paper all the workpiece processing links are carried out under the salt oxide bath formula A. In the initial production, the oxidized salt is added to the position of the crucible 1/2, and then the appropriate amount of oxidized salt is gradually added to raise the salt bath surface. An oxide is formed on the surface of the salt bath, which can improve the corrosion resistance [37-38]. The oxidized salt is shown in Figure 2.1(c).
Adjust salt: Adjust salt is used to adjust the concentration of cyanate in the salt nitride bath, and keep the concentration of active nitrogen atoms stable, so that nitrogen atoms can smoothly enter the workpiece surface, activate the salt nitride bath, improve the nitride speed. Adjusted salt will produce pungent odor when added to the nitriding furnace with a small amount of sputtering, which should be added slowly and in a small amount and fed by the feeder, as shown in Figure 2.1(b).
1.2 Cyanate concentration detection
The content of cyanate in salt bath directly affects the concentration of active nitrogen atoms and the nitriding rate, and in order to improve the thickness of infiltration layer, the concentration of cyanate in nitriding furnace should be measured every once in a while. In the detection process, silver nitrate as titration solution, potassium chromate as indicator, analysis steps:
(1) Dip an iron rod in a nitriding salt bath, crystallize into a solid, take 2g, put it in a beaker, ground it into powder, transfer it into a beaker, add 50ml distilled water, heat it at high temperature, and transfer it into a 100ml volumetric bottle.
(2) In order to remove the influence of carbonate in the salt bath, 25ml silver nitrate solution should be added to precipitate the carbonate in the solution, and then removed after filtration. The ionic reaction is shown in Equation (2.1).
![]()
(3) Take 20ml of salt solution to remove carbonic acid and add 3 drops of phenolic phthalein solution. The solution appears red. Then add silver nitrate titration solution to the solution until the red color fades and the color recovers to form white precipitate AgCNO. Record the volume of the solution reading V1 at this point.

(4) Take 20ml of the salt solution after reaction in (3), and add 3 drops of potassium chromate solution, continue to add slightly excessive silver nitrate solution, to produce white precipitated Ag[Ag(CN)2]. The excess silver nitrate solution was used to eliminate the potassium chromate indicator that had just been added, and was combined with it to form a reddish brown precipitate Ag2Cr2O4, which recorded a volume reading of V2 after the reaction. The ionic reaction was shown in equations (2.4) and (2.5).
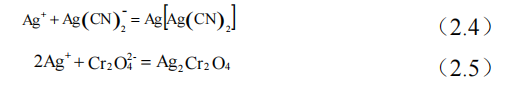
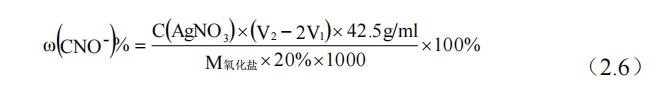
Preparation of test sample
In the experiment, 42CrMo steel materials in Shandong Liaocheng Hongao Metal Materials Co., LTD., in Anshan laser Industrial Park, the use of Taizhou integrated CNC machine tool manufacturing Co., LTD., the model of DK77 electric discharge CNC wire cutting machine tools (patent number: ZL012379654) cut 42CrMo bars into Φ38x5mm samples, and completed the cutting of subsequent experimental pieces. 618S Yuqing grinder was used to smooth the cutting surface of the specimen so that its roughness was reduced to less than Ra0.4. The specific equipment used is shown in Figure 2.2.

2. Experimental equipment
2.1 Experimental equipment for QPQ salt bath nitriding process
⑴ Ultrasonic cleaning machine: remove the stain on the surface of the specimen and play a cleaning role, as shown in Figure 2.3(a).
⑵ Preheating furnace: external heat crucible resistance furnace and its temperature control system. The furnace is equipped with national standard two-level thermocouple, and the electric furnace control cabinet model is KRJ-60. The preheating furnace is an air furnace used to dry the surface of specimens and raise the temperature to the normal working temperature.
Nitriding furnace: external heat crucible resistance furnace and its temperature control system. The furnace is equipped with national standard two-level thermocouple. The electric furnace control cabinet model is KRJ-60, equipped with or without paper recorder, the data of the whole process can be automatically recorded and printed out by computer. The distribution cabinet of nitriding furnace is equipped with a temperature alarm device to ensure safe production. The nitriding furnace is shown on the left in Fig.2.3 (b).
Oxidation furnace: an external heat crucible resistance furnace and its temperature control system. The furnace is equipped with a matching national standard two-level thermocouple. The oxidation furnace is shown on the right of Fig.2.3 (b).
There are 4 cleaning tanks with a size of 700mmX800mmX1000mm, which are filled with appropriate amount of water to facilitate the cleaning of specimens after nitriding and oxidation.

3. Heat treatment method
There are many methods for heat treatment of 42CrMo steel. In order to prevent surface cracks, 42CrMo steel nuts are annealed at 1050℃ and held for 6h, and then normalized at 860℃ [39]. During continuous cooling at high temperature, bainite is produced to refine the structure and improve the fatigue resistance of the workpiece [40]. The study on the influence of quenching and tempering state on the microstructure and impact toughness of 42CrMo steel shows that after quenching, the microstructure of 42CrMo steel is composed of lath martensite, strip and granular, which improves the hardness and wear resistance of 42CrMo steel [41-42]. Guo Shiqi et al. worked out 2h quenching at 840℃ + 3h tempering at 590℃ in the improvement of quenching process of 42CrMo steel double-row sprocket shaft, ensuring that the quenching hardness met the requirements [43].
The heat treatment furnace used in this paper is Pit-type resistance furnace, rated power of 4KW, rated temperature of 1000℃. The experimental workpiece adopts the heat treatment process of Anshan Weike Technology Co., LTD., China Three Metallurgical Group. The specific tempering process is 840℃ quenching and insulation, cooling in oil, and tempering at 590℃. After tempering, the hardness is less than or equal to 285HV, and the yield limit is less than 930MPa to improve the hardness and toughness of the specimen. The experimental procedure is as follows.
Rotate the power adjustment key to the right, when the temperature is adjusted to the appropriate temperature; According to the operation requirements of pneumatic, start running high temperature heating; At the end of time, rotate the power adjustment button to the left, and remove the specimen after the work is finished.
4. QPQ salt bath nitriding process
The specimen is polished, nitrized and oxidized. Nitriding and oxidation are the main processes of salt bath nitriding, and the general temperature is below 590℃ eutectoid transition temperature, so as to improve the production efficiency and increase the thickness of the permeability layer formed by the specimen. In this paper, the nitriding process parameter range [44-45] studied by Professor Rodeford is used for reference. The nitriding process range is as follows.
The preheating temperature is 350℃ to 400℃, and the time is 20 to 40min.
The nitriding temperature was 520℃ to 580℃, and the time was 30 to 180min.
The oxidation temperature is 350℃ to 400℃, and the time is 15 to 30min[44-45].
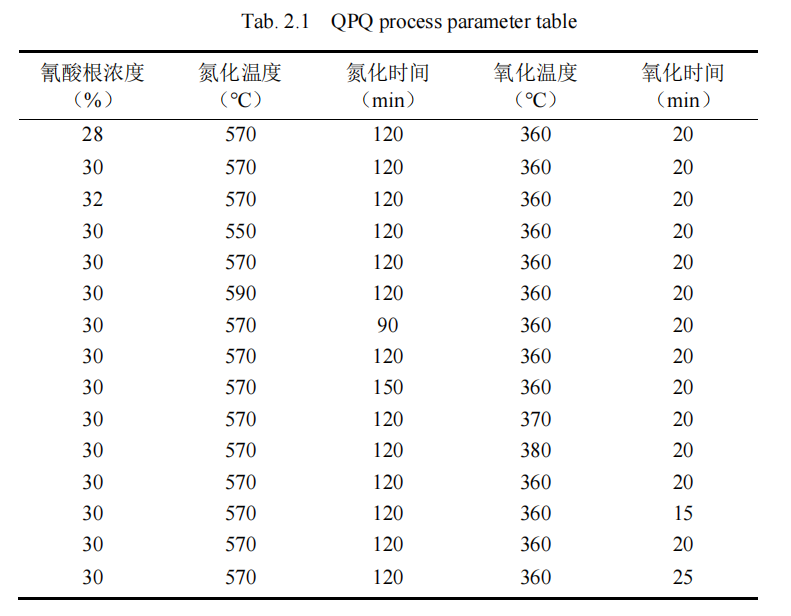
In this paper, according to the actual situation, the detailed design of a new QPQ salt bath nitriding process, the method is mainly divided into two parts, the first part is to analyze the impact of five process parameters on 42CrMo materials, the impact of the small process parameters abandoned; The second part is the use of orthogonal experiment table, through hardness experiment, metallographic experiment, XRD experiment, electron microscope scanning experiment, friction and wear experiment and electrochemical corrosion experiment, among which the electrochemical corrosion index is orthogonal analysis by multi-index comprehensive scoring method, and finally the experimental index conclusion is the overall analysis of the experimental results by multi-index comprehensive balance method. The optimal combination of process parameters and their main and secondary influences were obtained.
Firstly, the influence of a certain process parameter on compound depth and hardness of piston rod material was analyzed separately, and the influencing factors of QPQ salt bath nitriding process on piston rod were determined, and the corresponding influencing level was determined. The goal is to minimize the number of experiments without compromising the results. The specific experimental process parameters are shown in Table 2.1. Then, L9(33) orthogonal method, that is, three-factor and three-level orthogonal method, was used to conduct QPQ salt bath nitriding process on 42CrMo steel specimen, as shown in Table 2.2.
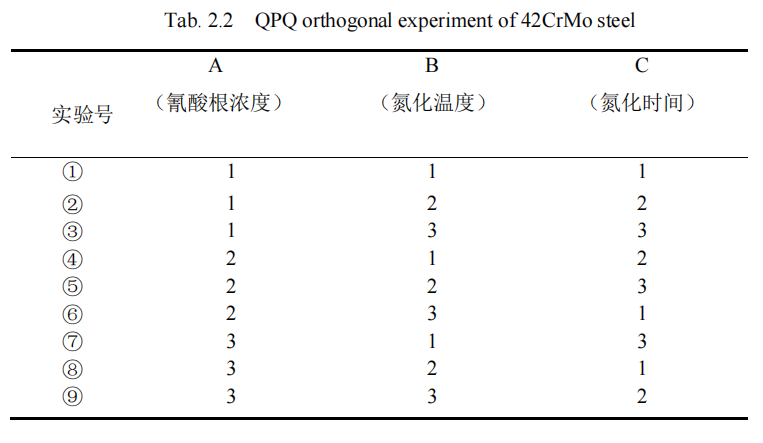
For Table 2.2, the specific 3 factors and 3 levels in the table are represented as follows:
Preheating: 380℃×20min;
Nitriding temperature (℃) : 550, 570, 590, corresponding to “1”, “2”, “3” in column B respectively;
Nitriding time (min) : 90, 120 and 150, corresponding to “1”, “2” and “3” in column C respectively;
Cyanate concentration (%) : 28, 30, 32, corresponding to “1”, “2”, “3” in column A respectively.
In the orthogonal calculation, the K value (see Chapter 5.1.2 in the fifth chapter) in the orthogonal result is different from the range, which indicates that when different levels of different factors change, the corresponding experimental index also changes. Usually, the value is calculated and analyzed to judge the influence degree of factors. The greater the change, the greater the influence, and the greater the influence on the experimental index. This also directly affects the conclusion of the experiment. When the range value is the maximum, it indicates that the influence degree of the factor is the greatest, that is, the factor is the main factor. Finally, the relative influence degree of each factor and the optimal scheme are obtained
5. Summary of this chapter
This chapter gives the experimental materials and introduces the corresponding experimental equipment. In this chapter, heat treatment processing method and QPQ salt bath nitriding process are formulated. The QPQ salt bath nitriding process is divided into two major experiments, and the selection table of process parameters and the orthogonal table of process parameters are given in order to obtain the optimal combination of process parameters and influence the primary and secondary.
6. Selection of orthogonal factors of QPQ salt bath nitriding process
According to Table 2.1, QPQ salt bath nitriding was performed on 42CrMo steel, and the effects of five different QPQ salt bath nitriding process parameters on the thickness and hardness of the permeability layer were studied. First determine a process parameter as the independent variable X, keep the other process parameters constant and unchanged under the premise of QPQ salt bath nitriding treatment of 42CrMo steel, by using the hardness tester to measure the hardness of the specimen, the thickness of the penetration layer is the core hardness +30HV hardness value of the corresponding depth of the penetration layer. The following is a detailed analysis of the process parameters on the influence of 42CrMo steel penetration layer thickness and hardness.
6.1 Influence of cyanate concentration on infiltration layer of 42CrMo steel
Under the condition that the cyanate concentration is changed within a certain range and other parameters are controlled, QPQ salt bath nitride treatment is carried out on 42CrMo steel specimen. After treatment, the hardness of the permeation layer is measured and the thickness of the permeation layer is obtained. Figure 3.1 shows the influence curve of different cyanate concentration on the microhardness of the permeation layer. FIG. 3.2 shows the influence curve of different cyanate concentration on penetration layer thickness.
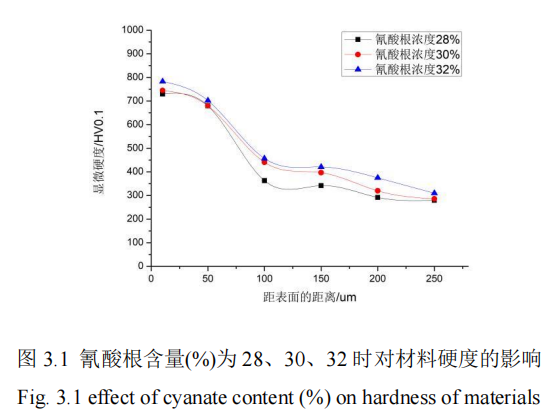
Figure 3.1 shows the relationship between the hardness of 42CrMo steel after nitriding and the percentage content of cyanate (28%-32%) of QPQ salt bath nitriding process parameters. The values of other process parameters are strictly controlled. The experiment shows that the hardness value of the material also increases gradually with the continuous increase of cyanate concentration. And the curve at 32% cyanate concentration is always higher than the curve at other concentrations. The maximum microhardness of the workpiece at 10um is 783HV, and the minimum hardness of the penetration layer is 310HV. In the figure as a whole, when the distance from the surface increases from 0um to 250um, the microhardness value of the matrix material decreases between 800HV and 250HV, and shows a fast decline trend before 100um. However, after 100um, the value decreases slowly. This is because nitrogen atoms enter the surface of the material in the way of diffusion during salt bath nitriding, so more nitrogen atoms are adsorbed on the surface of the material than in the core. Moreover, the core of the base material belongs to the quenching zone, which cannot form nitrogen-containing compounds, while active nitrogen atoms on the surface of the base material are more likely to form compounds with iron. So the surface hardness value is larger. The reason is that the concentration of active nitrogen atoms in the salt bath is much higher than the concentration of nitrogen atoms on the surface of the test piece at the beginning, so the diffusion rate is very fast, which also speeds up the formation rate of Fe nitride and greatly improves the surface hardness of the test piece. With the continuous increase of nitrogen atom concentration on the surface of the test piece, When the concentration of nitrogen atoms on the surface of the specimen is slightly higher and the concentration of nitrogen atoms inside the specimen is slightly higher, a small amount of Fe nitride will be formed. Therefore, with the increase of the distance from the surface, the hardness of the infiltration layer will decrease sharply until it reaches the hardness of the matrix. In the distance from the surface of the position can reach the core hardness, the core hardness values are 260HV, 263HV, 279HV, it can be seen that the core hardness is basically the same, so the independent variable cyanate concentration has an effect on the 42CrMo steel material penetration layer.
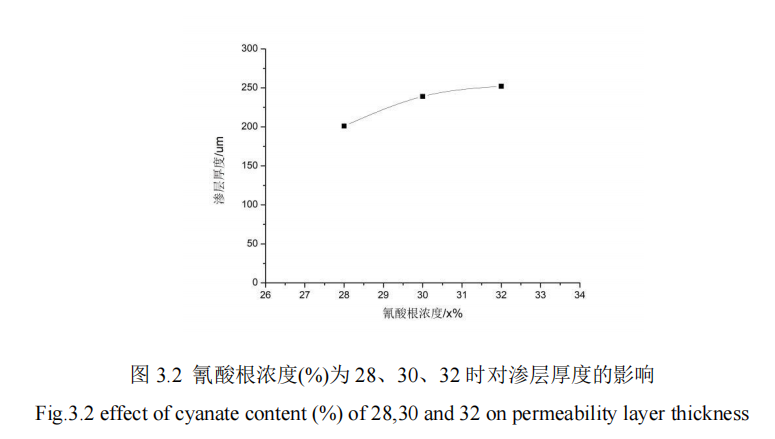
It can be concluded from FIG. 3.2 that when cyanate concentration is 28%, 30%, and 32%, other QPQ salt bath nitriding process parameters remain fixed, and with the continuous increase of cyanate concentration, the permeability layer thickness of 42CrMo steel material of vibration cylinder piston rod also gradually increases. Among them, the permeation layer thickness with cyanate concentration between 28%-30% changes faster than that with 30%-32%. When cyanate concentration is 32%, the material permeation layer thickness can reach 252um, which is nearly 50um higher than that when cyanate concentration is 28%, because the cyanate concentration keeps increasing. The concentration of active nitrogen atoms is higher than that of atoms on the workpiece surface, thus increasing the diffusion rate of N atoms; And the radius of N atom is only half of the radius of Fe atom, N atom is easier to combine with Fe atom inside 42CrMo piston rod, forming compound layer, improve the thickness of the infiltration layer, so the independent variable cyanate concentration has an effect on the QPQ salt bath nitriding process.
6.2 Influence of nitriding temperature on 42CrMo steel infiltration layer
In this section, the influence of nitriding temperature on the penetration thickness and hardness of 42CrMo steel is studied. When the nitriding temperature is 550℃, 570℃ and 590℃, QPQ salt bath nitriding treatment is carried out on 42CrMo steel under the condition that other process parameters remain unchanged. The hardness of specimens is measured by hardness measuring instrument. The hardness of specimens is measured from the hardness value of the specimen core to the thickness of the infiltration layer. FIG. 3.4 shows the influence curve of different nitriding temperatures on the thickness of infiltration layer.
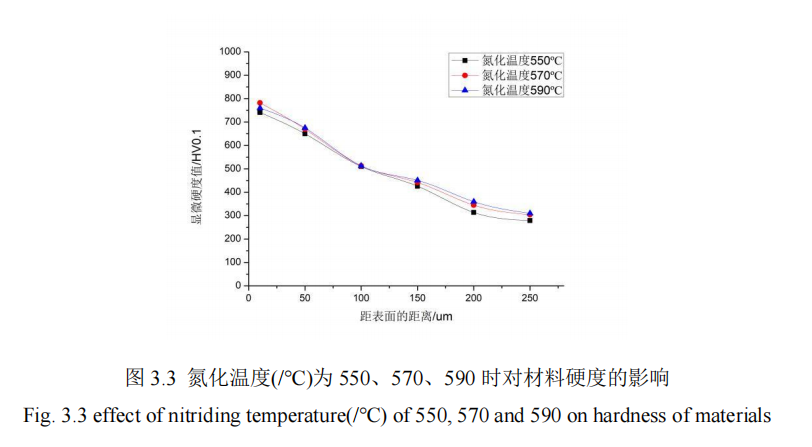
As can be seen from Figure 3.3, when the parameters of the other four groups of QPQ salt bath nitriding remain fixed and the nitriding temperature increases from 550℃ to 590℃, the conclusion can be drawn as follows: When the distance from the surface increases from 0um to 250um, the microhardness of the substrate decreases between 800HV and 260HV. The maximum microhardness of the substrate reaches 782HV and the minimum hardness of the penetration layer is 304HV when the nitridation temperature is 570℃ at 10um from the surface. The hardness values at 550℃ are always in the lowest state, and the hardness values at 590℃ are always in the highest state after 50um from the surface, and the micro-hardness values at different nitriding temperatures are in the same state at 100um from the surface. Combined with Figure 3.4, it can be seen that only when the nitriding temperature is 590℃ can the penetrative layer thickness of the material reach the maximum thickness of 254um. However, when the nitriding temperature is 10um away from the material surface, the microhardness of 590℃ is lower than that of 570℃. This is because a loose layer is generated on the surface of the material when the nitriding temperature is too high. The penetration layer thickness will increase but the hardness value will decrease. The overall downward trend of the curve is approximately the same. The hardness reaches the maximum at 10um away from the surface of the material, and the hardness of the core can be reached at a distance away from the surface. The core hardness values are 268HV, 273HV and 281HV, respectively. Therefore, the nitriding temperature has an effect on the hardness of 42CrMo steel substrate.
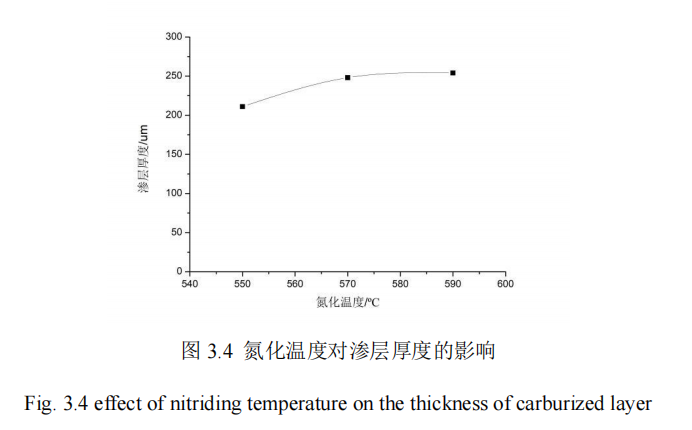
As can be seen from FIG. 3.4, when the parameters of the other 4 groups of QPQ salt bath nitriding process remain fixed and the nitriding temperature increases from 550℃ to 590℃, the permeation layer thickness of 42CrMo steel of the piston rod keeps increasing. The permeation layer thickness at 590℃ is nearly 50um higher than that at 550℃. The penetration layer thickness of nitriding temperature between 550℃ and 570℃ changes faster than that of nitriding temperature between 570℃ and 590℃. This is because the temperature of nitriding process can only be increased within a suitable range. Once excessive, the diffusion coefficient will also reach a peak, reducing the adsorption rate of active nitrogen atoms, and the increase of infiltration layer thickness is not obvious. There may also be loose, combined with economic benefits will be some more than worth the loss. Finally, when the nitriding temperature is 590℃, the material penetration layer thickness can reach 254um. Therefore, the parameter of nitriding temperature has an effect on the 42CrMo steel material penetration layer.
6.3 Influence of nitriding time on the infiltration layer of 42CrMo steel
In this section, the influence of nitriding time on the penetration thickness and hardness of 42CrMo steel is studied. When the nitriding time (/min) is 90, 120, 150, QPQ salt bath nitriding is carried out on 42CrMo steel under the condition that other process parameters remain unchanged, and the hardness of the specimen is measured by hardness measuring instrument. The hardness of the specimen is measured from the hardness value of the specimen heart to the thickness of the infiltration layer. Figure 3.5 shows the influence curve of different nitriding time on the hardness of the infiltration layer. FIG. 3.5 shows the influence curve of different nitriding time on the thickness of infiltration layer.
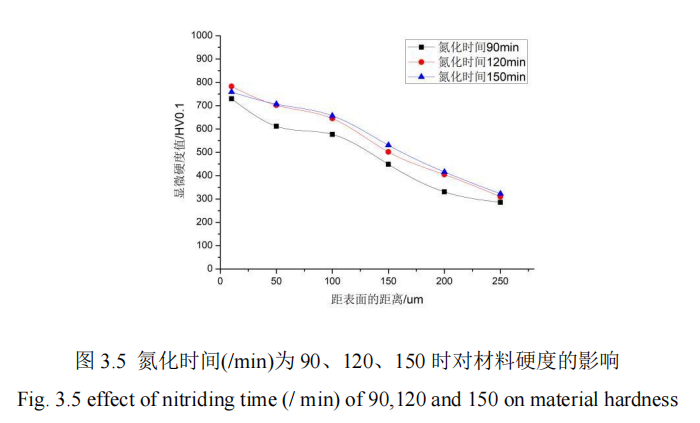
It can be seen from Figure 3.5 that the microhardness of the substrate increases when the nitriding time of the other four groups of QPQ salt bath is increased from 90min to 150min after the control variables are kept fixed. When the nitriding time is 90min, the hardness value is always in the lowest state. At 50um from the surface, the microhardness of 120min and 150min is basically the same. After 50um from the surface, the nitriding time is 150min, and at 10um from the surface, the nitriding time is 120min, the maximum microhardness is 783HV, and the minimum hardness of penetration layer is 310HV. However, combined with Figure 3.5, it can be seen that when the nitriding time is 150min, the penetrator thickness of the material can reach 262um. However, when the nitriding time is 10um away from the surface of the material, the microhardness value of the nitriding for 150min is lower than that of the nitriding for 120min. This is due to the excessive extension of the nitriding time. But the hardness value will decrease and loose layer structure will be produced. Generally speaking, the maximum hardness is still reached at the surface of the material, and the core hardness can be reached at the position far away from the surface. The core hardness values are 270HV, 279HV and 284HV at different nitriding times. According to experience, 120min is the best. Therefore, the nitriding temperature parameters have a great influence on the hardness of 42CrMo steel substrate.
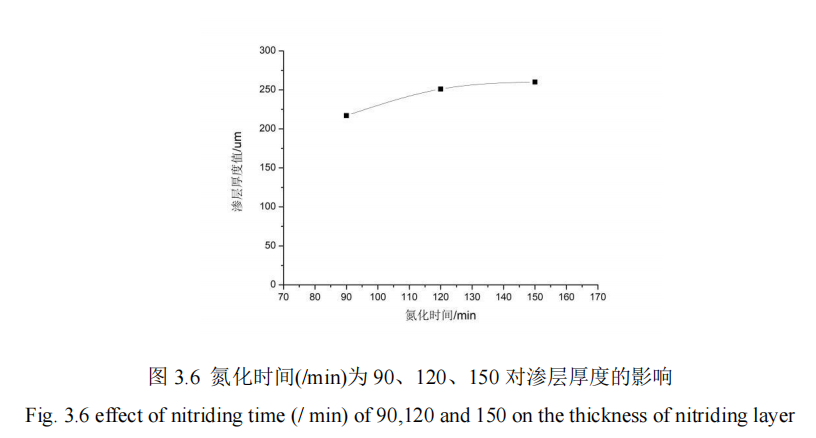
It can be concluded from FIG. 3.6 that when the parameters of the other 4 groups of QPQ salt bath nitriding process remain fixed and the nitriding time increases from 90min to 150min, the permeability layer thickness of the 42CrMo steel substrate of the piston rod of the vibration cylinder also increases continuously, and the overall permeability layer thickness changes are relatively moderate. The thickness variation of the infiltration layer in the first section is more obvious than that in the second section, which indicates that appropriate extension of nitriding time can improve the thickness of the infiltration layer. Once the concentration difference of active nitrogen atoms in the salt nitriding bath decreases, the formation capacity and quality of the infiltration layer will also become worse. From the perspective of economic benefits of production, 120min is more suitable for production. When the nitriding time is 150min, the thickness of the material penetration layer can reach 262um, which is nearly 50um higher than that when the nitriding time is 90min. Therefore, the nitriding time has an impact on the QPQ salt bath nitriding process of the piston rod substrate.
6.4 Influence of oxidation temperature on 42CrMo steel infiltration layer
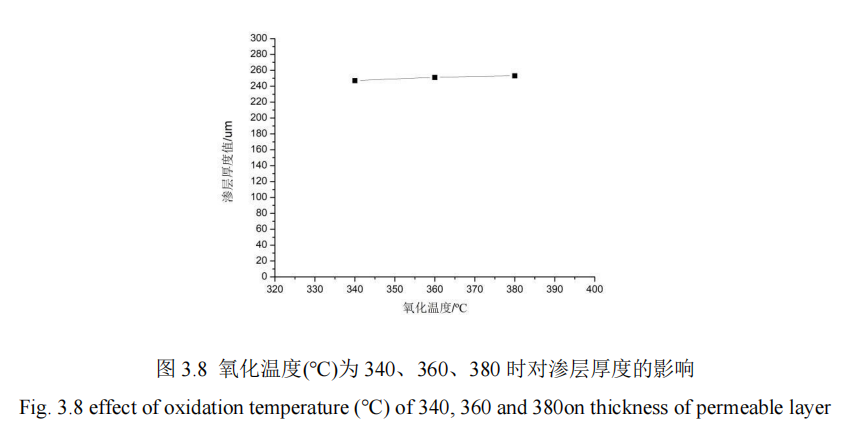
In this section, the influence of oxidation temperature change on the penetration thickness and hardness of 42CrMo steel is studied. For the oxidation temperature of 340℃, 360℃, 380℃, control other process parameters unchanged condition QPQ salt bath nitriding treatment of 42CrMo steel, using hardness measuring instrument to measure the hardness of specimens, from the hardness value of the specimen core to the thickness of the penetration layer, Figure 3.7 shows the influence of different oxidation temperature on the hardness of the penetration layer. FIG. 3.8 shows the influence curve of different oxidation temperatures on the thickness of infiltration layer.
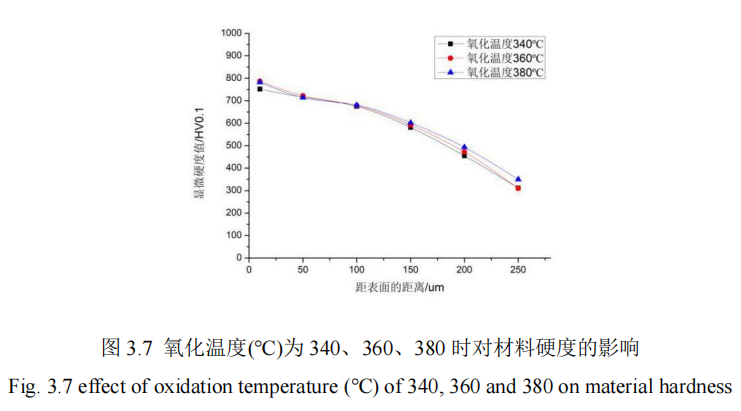
It can be concluded from Figure 3.7 that after fixing the process parameters of the other 4 groups of QPQ, the research finds that: When the oxidation temperature is 10um away from the surface, the penetration thickness of 360℃ and 380℃ is basically the same. When the oxidation temperature is 100um away from the surface, the microhardness values of the three temperature values are basically the same. After 100um away from the surface, the microhardness values of the nitridation temperature is 380℃ maintain the maximum value. However, in general, the microhardness of the three curves remains basically flat, which also indicates that the oxidation temperature has little influence on the hardness of the infiltration layer. Moreover, when the distance from the surface increases from 0um to 250um, the microhardness value also presents a decreasing state, which declines gently before 100um and rapidly after 100um. Moreover, the hardness reaches the maximum value at 10um on the surface of the material, which is about 787HV. The hardness of the core can be reached at a distance from the surface, and the core hardness values are 276HV, 281HV and 279HV, respectively. Therefore, the oxidation temperature has no effect on the QPQ salt bath nitriding process. It can be concluded from FIG. 3.8 that when the parameters of the other four QPQ salt bath nitriding process are fixed and the oxidation temperature increases from 340℃ to 380℃, the penetration layer thickness of the substrate surface does not change significantly, the difference is small, and the average value of its penetration layer thickness is about 250HV. However, after oxidation, the workpiece turned black, because the surface of the specimen produced oxide film, which can improve the oxidation resistance, but has little effect on the thickness and hardness value of the specimen, so the oxidation temperature has no effect on the QPQ salt bath nitride process.
6.5 Influence of oxidation time on the infiltration layer of 42CrMo steel
In this section, the influence of nitriding time on the penetration thickness and hardness of 42CrMo steel is studied. When the nitriding time is 15min, 20min, 25min, QPQ salt bath nitriding treatment is carried out on 42CrMo steel under the condition that other process parameters remain unchanged, and the hardness of specimens is measured by hardness measuring instrument. The hardness of specimens is measured from the hardness value of the specimen core to the thickness of the infiltration layer. Figure 3.9 shows the influence curve of different nitriding time on the hardness of the infiltration layer. FIG. 3.10 shows the influence curve of different nitriding time on the thickness of infiltration layer.
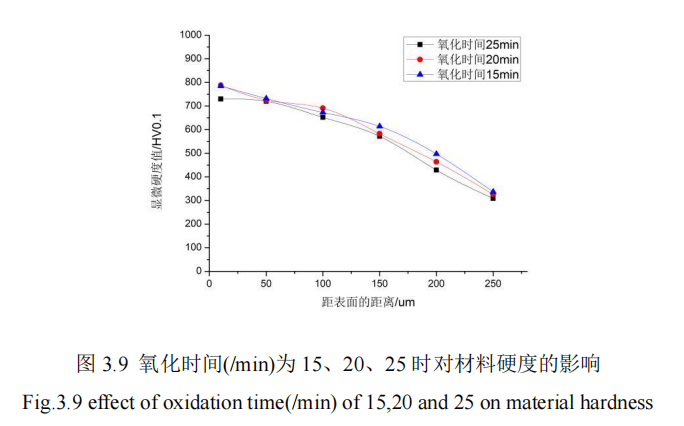
It can be concluded from Figure 3.9 that: Under the condition that the other four groups of process parameters are constant, the decline trend of the microhardness curve of the material is basically consistent with the increase of oxidation time. The surface hardness values of the specimens with nitriding time of 15min and 20min at the distance of 10um from the surface are basically the same, which has little influence on the hardness of the penetration layer. The microhardness values of the three oxidation times are also consistent. In general, when the oxidation time is between 15min and 20min, the hardness of the material surface reaches the maximum value, about 788HV. In the distance from the surface of the position can reach the core hardness, different oxidation time, the core hardness values are 281HV, 284HV, 272HV, so the oxidation time parameter has no effect on the hardness value of the vibration cylinder piston rod 42CrMo steel.
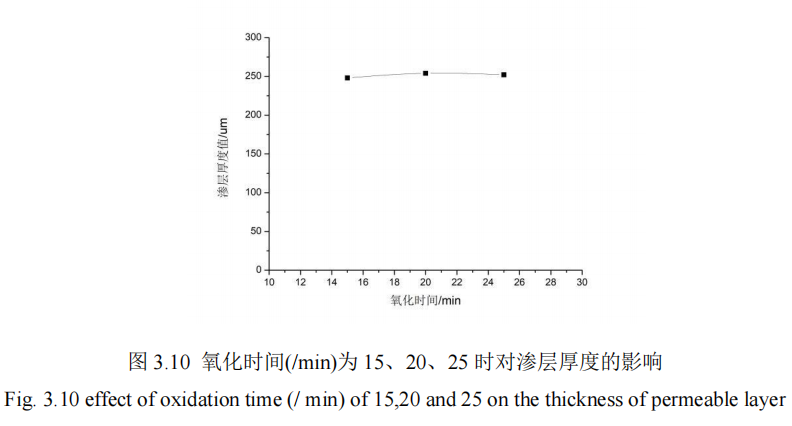
It can be concluded from Figure 3.10 that: The other 4 groups of QPQ salt bath nitriding process parameters remained fixed, the oxidation time of the independent variable increased from 15min to 25min, the permeation layer thickness of 42CrMo steel matrix material was basically stable, and the average value of the permeation layer thickness was about 251HV, the specimen was still black after processing. However, it does not significantly increase the penetration layer thickness of specimens, so the oxidation time process parameters have no effect on the penetration layer thickness of 42CrMo steel.
7.Summary
This chapter specifically studies the influence of five commonly used QPQ salt bath nitriding process parameters on the thickness and hardness of the permeability layer of 42CrMo steel of the vibration cylinder piston rod. The specific hardness value is obtained by Vickers hardness instrument, and the depth corresponding to the core hardness +30HV is the thickness of the permeability layer, and the hardness test data is processed by Origin mapping software. Two-dimensional curves of penetration layer thickness and hardness are obtained in detail, and corresponding two-dimensional graphs of the five process parameters are shown as follows: With the increase of concentration and temperature in the salt bath, the extension of the process time, the thickness of the infiltration layer is increasing, the Vickers hardness of the specimen is also gradually increased, and through analysis, cyanate concentration, nitriding temperature, nitriding time three kinds of QPQ salt bath nitriding process parameters are more important, on the piston rod 42CrMo steel surface infiltration layer thickness and hardness of greater influence, Moreover, the hardness reaches the maximum at 10um away from the workpiece surface, where the total thickness of the permeating layer is not proportional to the hardness of the permeating layer. This is because excessive increase of nitriding temperature and time will produce loose layer on the surface of the specimen and reduce the surface hardness of the specimen. The effect of different oxidation temperature and oxidation time process parameters on the specimen is basically the same. Only an oxide film is formed on the surface of the specimen, and the passivation surface has no obvious effect on the thickness and hardness of the seepage layer on the surface of the 42CrMo steel material of the vibration cylinder piston rod.

Learn More :
Gas Nitrocarburizing And Post Oxidation Processes For Shift Shaft 40CrH Steel Carbon Classification Of Carburizing Heat Treatment Carburizing, Carbonitriding Gear Heat Treatment Methods

Contact us
Your email address will not be published. Required fields are marked *