Effect of Annealing Temperature on Microstructure and Soft Magnetic Properties of FeGaGeBCu(P) Amorphous Nanocrystalline Alloy
Fe76Ga5Ge5B13-xPxCu1 (x=0, 3, 5, 7) alloy strips were prepared by single-roll spin quenching, and the effects of annealing temperature on the crystallization behavior, structural evolution and magnetic properties of the alloy were studied. The results show that the replacement of P element with B element reduces the amorphous formation ability of the alloy, but improves the thermal stability of the secondary crystallization phase. At the same time, on the one hand, the addition of P refines the size of the nanocrystals and reduces the coercive force of the alloy. The coercive force of the alloy with a P content of 7% (atomic fraction) is 1.77 A/m after annealing at 425 °C; On the one hand, the valence electrons of P transfer to Fe in the residual amorphous phase, which reduces the magnetic moment of Fe and reduces the saturation magnetic induction of the alloy. In addition, when the P content is higher than 5% (atomic fraction), the grains on the surface of the alloy strip near the surface exist with the (200) crystal plane parallel to the strip surface, while the (110) crystal plane is still parallel to the strip surface inside the alloy, surface exists.
In recent years, iron-based amorphous nanocrystals have attracted widespread attention due to their excellent soft magnetic properties, such as high magnetic permeability, low coercive force and low core loss [1-3]. In today’s global energy crisis and technological progress, the development of electrical equipment requires the development of miniaturization and energy saving [4], which requires iron-based amorphous nanocrystals to have higher saturation magnetic induction Bs and lower coercivity. Force Hc. There are two ways to increase Bs: (1) directly increase the content of Fe; (2) add elements that can increase the magnetic moment of Fe such as Ga, Ge, Co, etc. [5-7]. Directly increasing the Fe content is the most effective way to increase the Bs of the alloy, but a large number of studies have shown that [8-9], due to the increase of the Fe content and the decrease of the metalloid element content, the as-quenched alloy is prone to partial crystallization, which requires The alloy has a high heating rate during the annealing process, otherwise it is prone to coarse grains and uneven grain distribution, which is not conducive to the improvement of soft magnetic properties. For the second method, the magnetic moment of Fe in the alloy can be increased by adding Ga, Ge, Co, etc. [5-7], thereby increasing the Bs of the crystallized phase. At the same time, due to the doping of various elements, the alloy has a certain Amorphous forming ability, so as to prepare alloy strip with higher degree of amorphous. Studies have shown that Ga can form a solid solution with Fe, which makes the iron-based amorphous alloy precipitate α-Fe (Ga) nanocrystalline phase during crystallization, which improves the Bs of the alloy [6,10]. It should be noted that most iron-based amorphous nanocrystals are obtained by annealing the amorphous matrix, which consists of two parts: nanocrystals and residual amorphous phase. Nanocrystals perform ferromagnetic exchange coupling through the amorphous layer, making them have excellent soft magnetic properties [11]. Therefore, the Bs of the alloy is composed of two parts, namely, the magnetic induction intensity Bsc of the nanocrystal and the magnetic induction intensity Bsa of the residual amorphous phase, which can be expressed as Bs=Bsc*Vc/V+Bsa*Va/V (Vc/V represents the volume of the crystallized phase Fraction, Va/V represents the volume fraction of residual amorphous phase)[12]. Therefore, in order to further increase the Bs of the alloy, it is also necessary to pay attention to the magnetic induction intensity Bsa of the residual amorphous phase. Non-metallic elements such as Si, B, P, C, etc. are usually used as additive elements to improve the amorphous formation ability of the alloy. Since P, B, and C are insoluble in Fe, they remain in the residual amorphous phase during the crystallization process of the alloy. , has a certain influence on Bsa, among which the influence of P is the most obvious[13-16]. For example, after adding P element to Fe83.3Si4Cu0.7B12-xPx and Fe83.3Si2B13-x-PxC1Cu0.7, the Bs of the alloy decreases, but the mechanism is not yet clear.
According to our previous research, the Fe76Ga5Ge5B13Cu1 alloy was prepared by adding Ga and Ge, and the Bs was increased compared with the alloy with the same Fe content. , by replacing B in Fe76Ga5Ge5B13Cu1 with P, we studied the effect of annealing temperature on the microstructure and soft magnetic properties of Fe76Ga5Ge5Ge5B13-xPxCu1 (x=0, 3, 5, 7) alloys, and analyzed the annealing temperature on the crystallization behavior, Structural evolution and mechanism of influence on magnetic properties.
1.Experiment
1.1 Preparation of FeGaGeBCu(P) amorphous ribbons
In the experiment, high-purity raw materials Fe, Ga, Ge, B, Cu (99.99% (mass fraction)) and PFe (P content is 25.23% (mass fraction)) master alloy were selected, and the vacuum electric arc furnace was used in the argon atmosphere. Next, the Fe76-Ga5Ge5B13-xPxCu1 (x=0, 3, 5, 7) master alloy was smelted and smelted three times to ensure the uniformity of the composition of the master alloy. An alloy strip with a width of about 2 mm and a thickness of about 20 μm was prepared by the single-roll spin quenching method, and the rotation speed of the roll was about 29.9 m/s. Rapid annealing was carried out in a vacuum annealing furnace, the temperature range was 375-500 °C, the heating rate was 100 °C/min, and the holding time was 1 min.
1.2 Properties and characterization of samples
The structure of the as-quenched and annealed amorphous strips was detected by a MiniFlex600 X-ray diffractometer (Cu-Kα radiation source, λ = 0.15418 nm) and a transmission electron microscope (TEM, JEM2100F). Non-isothermal DSC curves were measured on a NETZSCH-STA449C differential or scanning calorimeter under a nitrogen atmosphere with a heating rate of 35 °C/min. The saturation magnetic induction Bs was measured with a vibrating sample magnetometer (VSM, Versalab), and the coercive force was detected with a MATS-2010S B-H instrument. The sample size was 50mm×2mm×20μm.
2. Results and discussion
2.1 Amorphous formation and thermodynamic properties research Figure 1 is the XRD pattern of the free surface of the as-quenched Fe76Ga5Ge5B13-xPxCu1 (x=0, 3, 5, 7) alloy.
It can be seen from Figure 1 that the quenched x=0 alloy has a diffuse scattering peak around 2θ=45°, indicating that the x=0 alloy is an amorphous structure. However, x=3 alloy has sharp peaks at 2θ=45° and 65°, indicating that the alloy has been partially crystallized, and the degree of crystallization is relatively high. The quenched x=5 alloy and x=7 alloy have diffuse scattering peaks around 2θ=45°, and the (200) plane of α-Fe is a sharp peak at around 2θ=65°, which also shows that the quenched x=5 alloy and x = 7 alloy part of the crystallization. The crystal plane detected in XRD is (200) instead of (110), because when the P content is high, the (200) plane of the grain is parallel to the strip surface, which is easy to detect [14,17-18]. In summary, after P element replaces B element, the amorphous formation ability of Fe76Ga5Ge5B13-xPxCu1 (x=3, 5, 7) alloy becomes worse. The solid-liquid coexistence temperature range experienced by the alloy during rapid quenching from the molten state to a strip is larger, and it is easier to nucleate and crystallize.
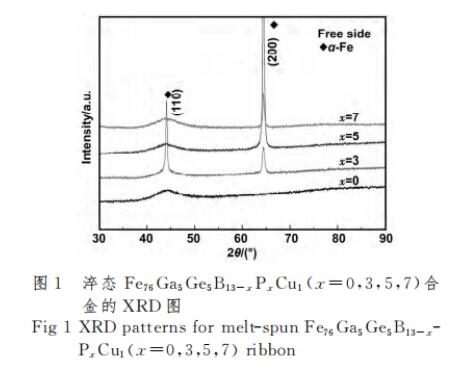
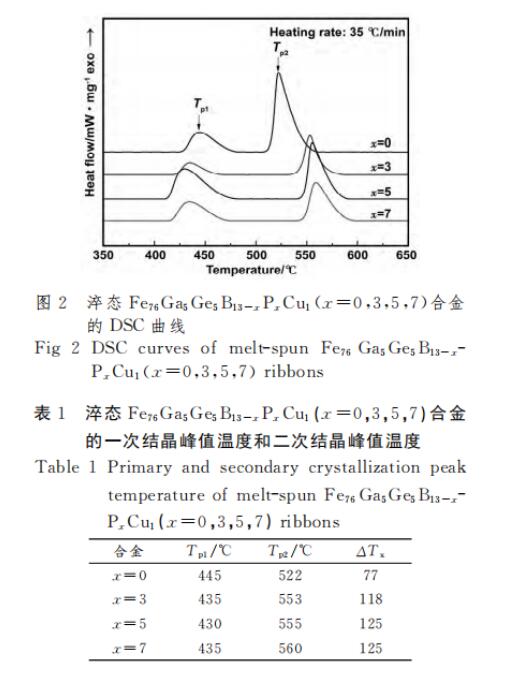
Figure 2 is the non-isothermal heating DSC diagram of as-quenched Fe76Ga5Ge5B13-xPxCu1 (x=0, 3, 5, 7) alloy, all DSC curves have two exothermic peaks, the first exothermic peak is α-Fe The second exothermic peak is the precipitation of FeB(P) phase[10,19]. It can be seen from the figure that after the addition of P, the first peak temperature Tp1 shifts to the left. This is because after the addition of P, there is a small amount of crystallization phase in the alloy, which provides a nucleation point for the precipitation of the primary crystal phase. In addition, P can form Cu-P clusters with Cu[20], which increases the nucleation rate and makes the primary crystal phase easier to precipitate. The second peak temperature Tp2 shifts to the right, because P is insoluble in Fe and does not participate in the precipitation of the primary crystal phase, but exists in the residual amorphous phase, thus improving the thermal stability of the residual amorphous phase. Table 1 shows the peak temperature of the two exothermic peaks and the difference between the two crystallization exothermic peak temperatures ΔT=Tp2-Tp1. After adding P, ΔT increases, which is beneficial to expand the crystallization temperature of α-Fe and reduce the alloy’s crystallization temperature. Annealing sensitivity, and with the increase of P content, ΔT gradually tends to be stable.
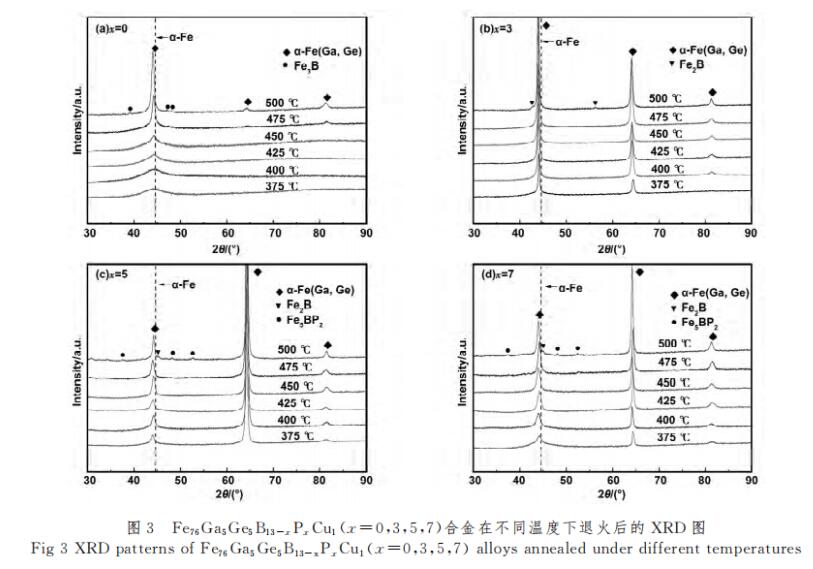
The Fe76Ga5Ge5B13-xPxCu1 (x=0, 3, 5, 7) alloy was annealed in a vacuum annealing furnace at a temperature of 375-500 °C and a holding time of 1 min. Figure 3 is the XRD pattern after annealing at different temperatures. The x=0 alloy begins to precipitate α-Fe phase when the annealing temperature is 425 °C, as shown in Figure 3(a). And x=3, 5, 7 alloys all began to precipitate α-Fe phase at 375 °C, as shown in Figure 3(b)-3(d), which also corresponds to the DSC diagram in Figure 2, that is, the primary crystal The precipitation temperature of the phase decreases. In addition, the diffraction peak angles of the primary crystal phase of the alloy are smaller than those of the pure α-Fe phase. This is because Ga and Ge can dissolve in α-Fe to form a substitutional solid solution, and the atomic radius of Ga and Ge is larger than that of Fe, resulting in the formation of α-Fe. According to the Bragg equation 2dsinθ=nλ (where d is the interplanar spacing, θ is the diffraction angle, n is the reflection order, and λ is the X-ray wavelength), the diffraction angle decreases accordingly. , which proves that the primary crystal phase in the alloy is α-Fe (Ga, Ge). When the temperature rises to 500 ℃, all four alloys precipitate hard magnetic phase, x=0 alloy precipitates Fe3B phase, x=3 alloy precipitates Fe2B phase, x=5, x=7 alloy precipitates Fe2B phase and Fe5BP2 phase.
2.2 Research on soft magnetic properties and microstructure
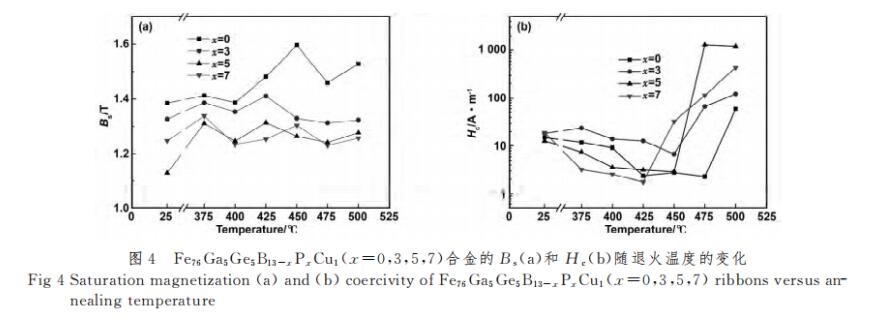
Figure 4(a) shows the change of Bs of Fe76Ga5Ge5B13-xPxCu1 (x=0, 3, 5, 7) alloy with annealing temperature. It can be seen from the figure that when the annealing temperature is 425 ℃, x=3, 5 alloy Bs reaches the maximum value, which are 1.41 and 1.31T respectively. When the annealing temperature is 450 ℃, x=0, Bs of alloy 7 reaches the maximum value, which are 1.6 and 1.3T respectively. On the whole, with the increase of P content, the saturation magnetic induction intensity Bs of the alloy decreases gradually. Since P is insoluble in Fe and mainly exists in the residual amorphous phase and around α-Fe, studies have shown that metalloids can reduce the magnetic moment of Fe[21], after adding P, due to the Fermi level EF of Fe The EF of P is lower, and the EF of P is higher, so that the valence electrons of P transfer to Fe until the EF of the two are equal, as shown in Figure 5. The source of the magnetic moment of Fe is mainly the difference between the upper spin and lower spin electrons in the d-layer of extranuclear electrons. Since the lower spin state electrons are not full, the filling of valence electrons from P causes the lower spin electrons in the d-layer of Fe. The number increases, while the total number of up-spin electrons decreases, and at the same time reduces the magnetic moment of Fe and Bsa, thereby reducing the Bs of the alloy. Figure 4(b) shows the change of Hc of Fe76Ga5Ge5B13-xPxCu1 (x=0,3,5,7) alloy with annealing temperature. In the range of annealing temperature of 375-500 ℃, the alloy showed a trend of “decrease-increase”. When x=0 alloy reaches the minimum value of Hc at 475°C, it reaches 2.26A/m; when x=3 alloy reaches the minimum value of Hc at 450°C, it reaches the minimum value of 6.83A/m; The lowest value is 2.86A/m, and when x=7 alloy is at 425 ℃, Hc reaches the lowest value of 1.77 A/m. For x=0 alloy, when the annealing temperature is lower than 425 °C, the alloy is in a state of structural relaxation, annealing releases the internal stress of the alloy, reduces the stress-induced anisotropy of the alloy, and reduces Hc. At ~475 °C, nanocrystals are precipitated, and the anisotropy of the magnetocrystals is averaged, so Hc further decreases with the increase of annealing temperature. When the annealing temperature is 475 ℃, the grain size of the nanocrystals is the smallest, because the relationship between the grain size and the coercive force follows [10] Hc∝D6, so the Hc is the lowest. When the annealing temperature is higher than 475 °C, the nanocrystalline grains grow gradually, which makes Hc gradually increase. When the annealing temperature is 500 ℃, the sudden increase of Hc is due to the precipitation of Fe3B hard magnetic phase. The coercivity of x=3, 5, 7 alloys shows a trend of “decrease-increase”, the principle is consistent with that of x=0 alloys. Compared with the x=0 alloy, the Hc of the x=5 and x=7 alloys decreased gradually. This is because P is insoluble in Fe, and Cu-P clusters will be formed during the process of stripping, providing nucleation sites for α-Fe. P is enriched between α-Fe and the matrix, and its enrichment stabilizes the amorphous matrix and inhibits the growth of α-Fe[20,22]. So that the size of the nanocrystals is reduced, so x = 7 alloy Hc minimum. However, the Hc of the x=3 alloy is the largest, because the as-quenched alloy has been partially crystallized, and the crystallized volume fraction is relatively large, the grain size increases rapidly after annealing, and finally the coarse grain reduces the iron density between the nanocrystals. Magnetic coupling exchange effect, resulting in a larger Hc.
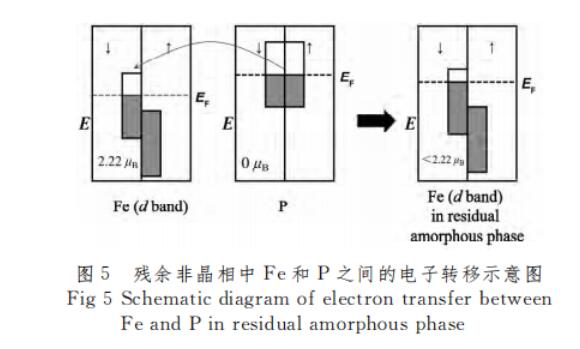
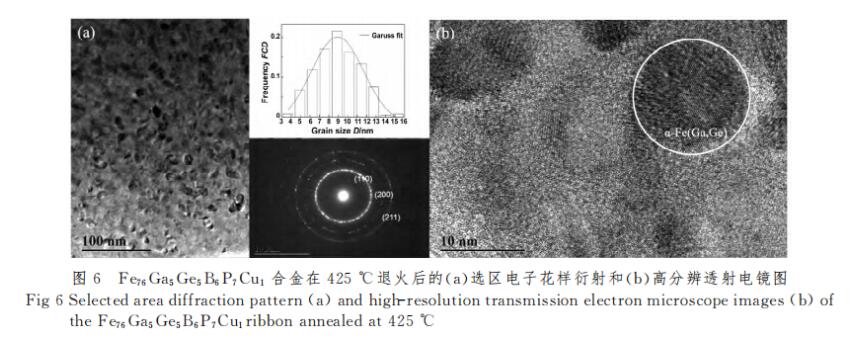
Figure 6 is the TEM image of the x=7 alloy after annealing at 425 °C. It can be seen from Figure 6(a) that the grains in the alloy are round and uniform in size, the average grain size is about 9nm, and the crystallization volume fraction is reasonable, which is conducive to the ferromagnetic coupling exchange between nanocrystals. Figure 6(b) is a high-resolution image, from which it is further confirmed that the grain size is around 9nm. In the selected electron diffraction pattern in Figure 6(a), the (110) crystal plane is the most obvious, and the (200) crystal plane is weaker. Since the TEM sample strips are thinned on both sides during the fabrication process, the structure in the figure is the strip This means that the (200) crystal plane parallel to the surface of the strip in XRD is only at the shallower position of the strip, and the center of the strip still exists with the (110) crystal plane parallel to the surface of the strip.
3.Conclusion
The effects of replacing B elements with P elements on the alloy Fe76-Ga5Ge5B13-xPxCu1 (x = 0, 3, 5, 7) amorphous formation ability, thermal stability, and soft magnetic properties were studied. The results show:
(1) The addition of P element leads to partial crystallization of the quenched alloy, and the precipitation temperature of the primary crystal phase decreases, which slightly reduces the amorphous formation ability of the alloy, but increases the precipitation range of the α-Fe (Ga, Ge) phase.
(2) When the P content is 7% (atomic fraction), the optimal Hc is 1.77A/m after annealing at 425 ℃, at this time, the Bs is 1.25T, and the grain size of the alloy is about 9nm. (3) P addition causes the valence electrons of P in the residual amorphous phase of the alloy to transfer to Fe, resulting in the decrease of Fe magnetic moment and Bs. In addition, when the P content is higher than 5% (atomic fraction), the grains close to the surface exist with the (200) crystal plane parallel to the surface of the strip, while the inside of the alloy still exists with the (110) crystal plane parallel to the surface of the strip.

Several Matters Needing Attention In Gas Nitriding Process Study on QPQ Nitriding Process of 42CrMo Piston Rod Gas Nitrocarburizing And Post Oxidation Processes For Shift Shaft 40CrH Steel


Contact us
Your email address will not be published. Required fields are marked *